

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-Y (Homo sapiens) | BDBM50535590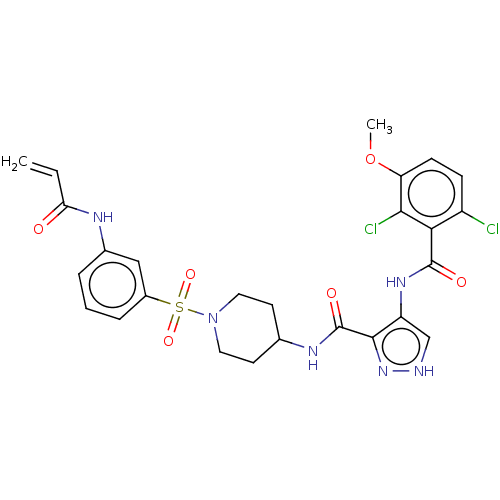 (CHEMBL4563703) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50193013 (Duvelisib | INK-1147 | INK-1197 | IPI-145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535569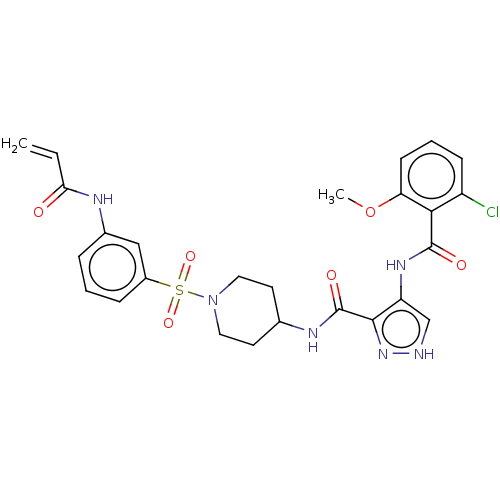 (CHEMBL4535078) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535520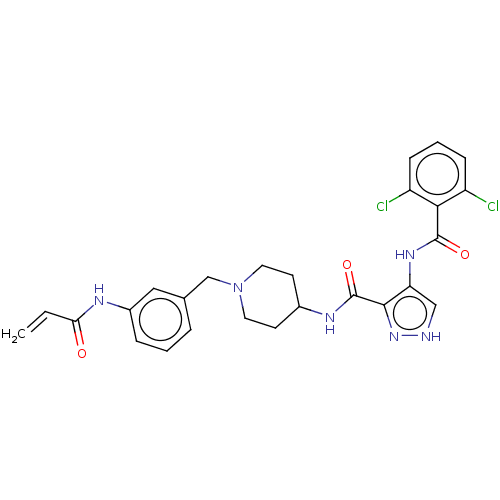 (CHEMBL4560035) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535547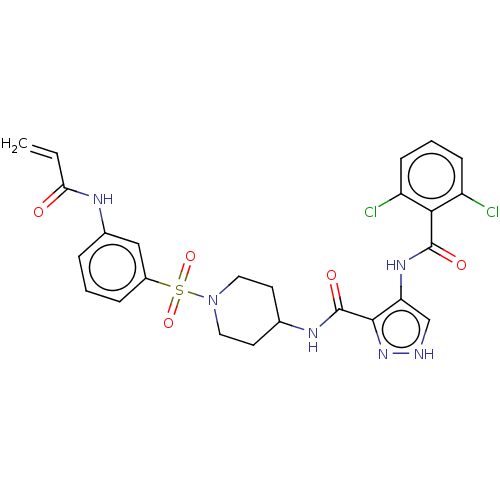 (CHEMBL4522564) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase DCLK1 (Homo sapiens (Human)) | BDBM50337136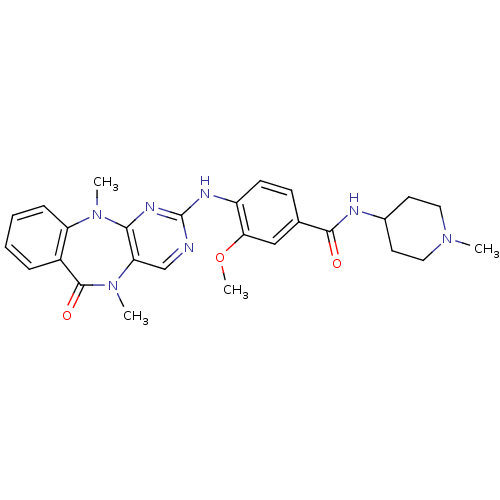 (4-((5,11-dimethyl-6-oxo-6,11-dihydro-5H-benzo[e]py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535532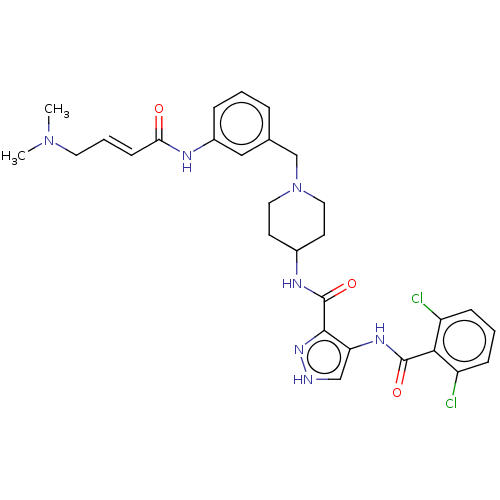 (CHEMBL4534124) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50193032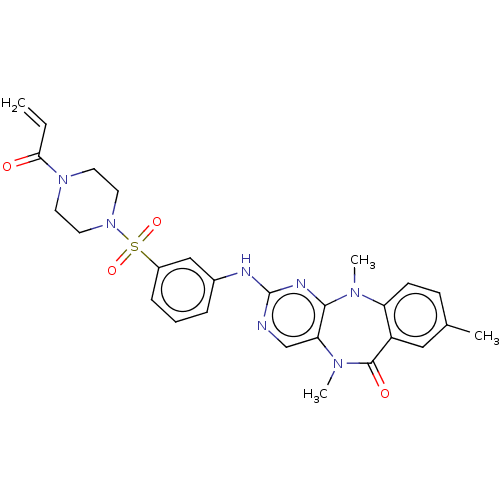 (CHEMBL3979343 | US11155556, No. 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535523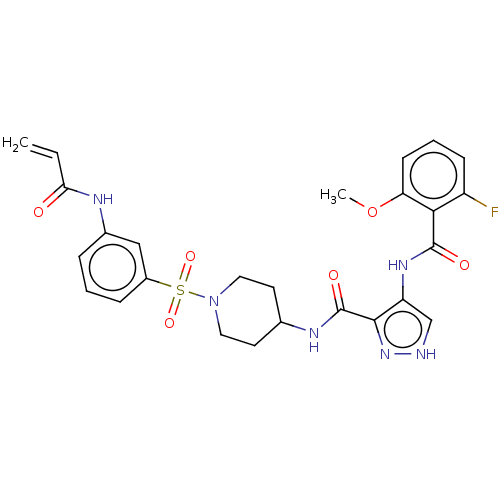 (CHEMBL4446451) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50193014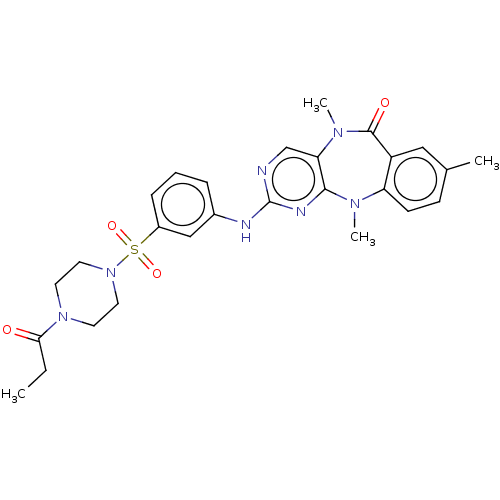 (CHEMBL3942643 | US11155556, No. 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535591 (CHEMBL4522598) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase C2 domain-containing subunit gamma (Homo sapiens (Human)) | BDBM50193032 (CHEMBL3979343 | US11155556, No. 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase C2 domain-containing subunit gamma (Homo sapiens (Human)) | BDBM50193014 (CHEMBL3942643 | US11155556, No. 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase C2 domain-containing subunit gamma (Homo sapiens (Human)) | BDBM50193031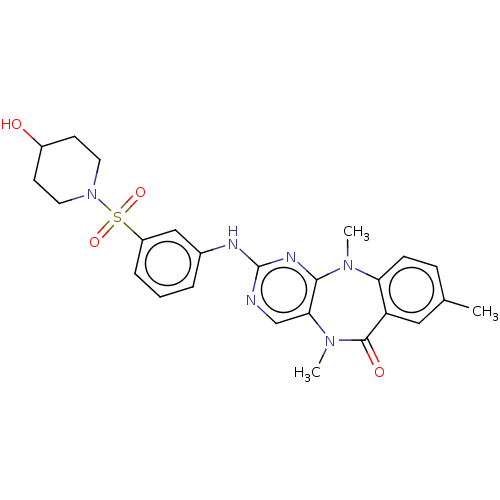 (CHEMBL3959351 | US11155556, No. 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50193017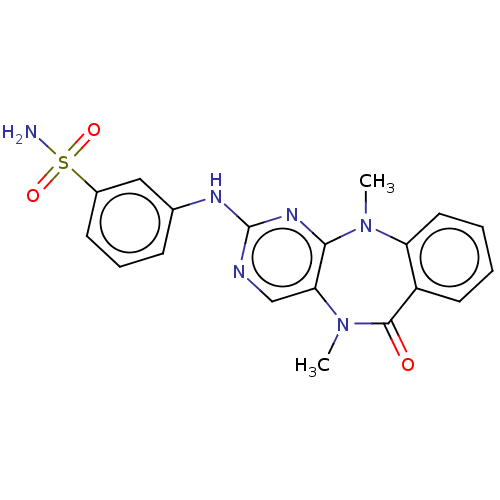 (CHEMBL3904942 | US11155556, No. 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase DCLK1 (Homo sapiens (Human)) | BDBM50539936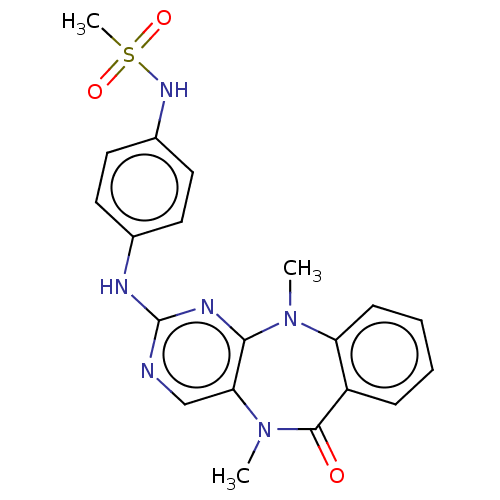 (CHEMBL4646447) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM150175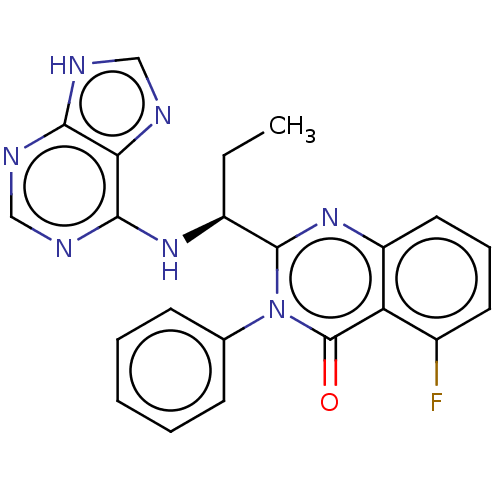 (US8980901, 107 | US9149477, Compound 107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase DCLK1 (Homo sapiens (Human)) | BDBM50539946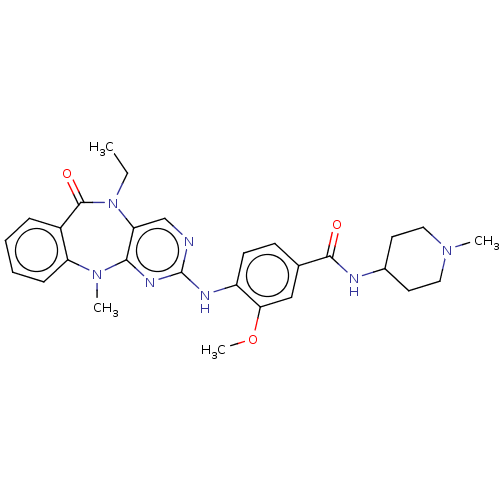 (CHEMBL4643632) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50193017 (CHEMBL3904942 | US11155556, No. 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human Aurora kinase A (E122 to K401 residues) expressed in mammalian expression system by Z'LYTE assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50193031 (CHEMBL3959351 | US11155556, No. 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535546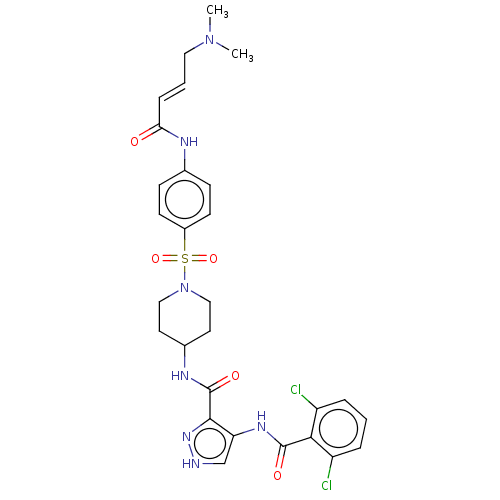 (CHEMBL4515515) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50193032 (CHEMBL3979343 | US11155556, No. 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50193013 (Duvelisib | INK-1147 | INK-1197 | IPI-145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50193032 (CHEMBL3979343 | US11155556, No. 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535571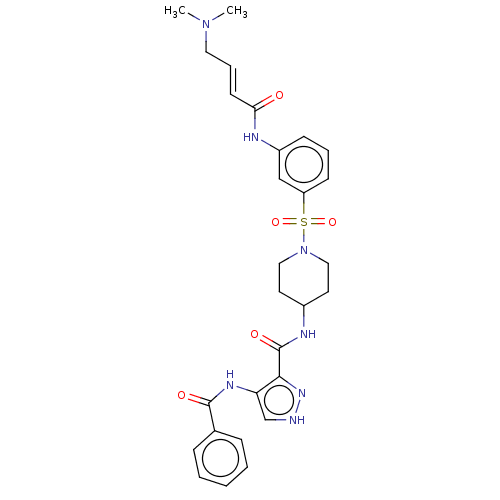 (CHEMBL4530961) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase DCLK1 (Homo sapiens (Human)) | BDBM50539943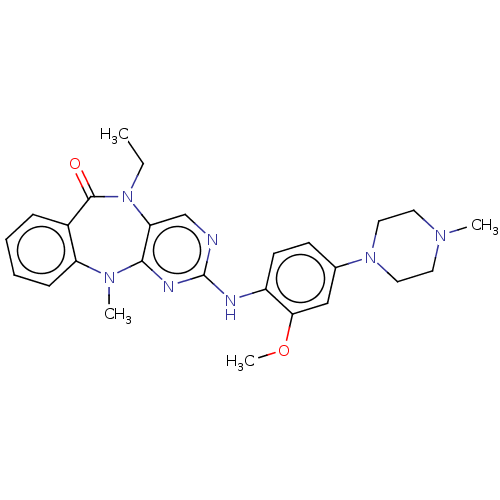 (CHEMBL4634326) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535535 (CHEMBL4542127) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase DCLK1 (Homo sapiens (Human)) | BDBM50539937 (CHEMBL4636475) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535581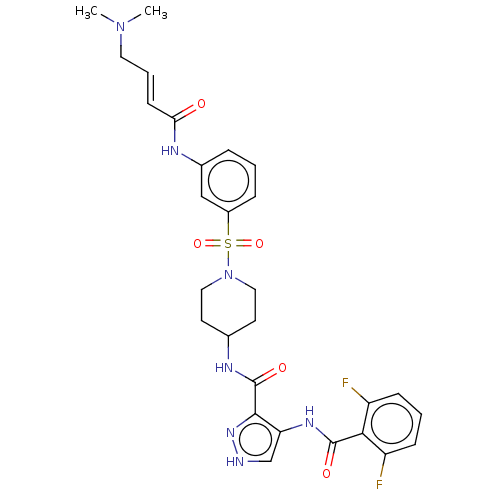 (CHEMBL4527767) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50193030 (CHEMBL3950648 | US11155556, No. 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase DCLK1 (Homo sapiens (Human)) | BDBM50539941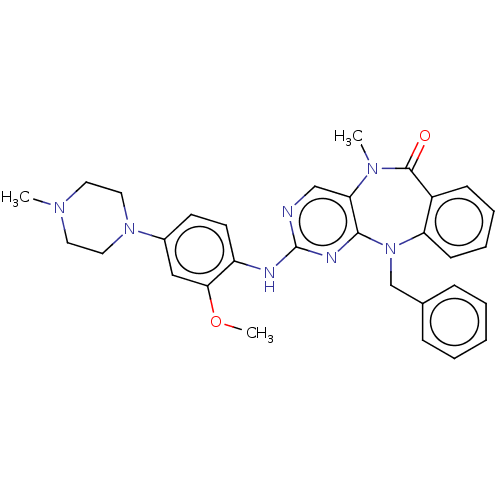 (CHEMBL4645267) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50193017 (CHEMBL3904942 | US11155556, No. 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50193017 (CHEMBL3904942 | US11155556, No. 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase C2 domain-containing subunit gamma (Homo sapiens (Human)) | BDBM50193017 (CHEMBL3904942 | US11155556, No. 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50193017 (CHEMBL3904942 | US11155556, No. 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50193030 (CHEMBL3950648 | US11155556, No. 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human Aurora kinase B (D25 to A303 residues) expressed in mammalian expression system by Z'LYTE assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50193017 (CHEMBL3904942 | US11155556, No. 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50193017 (CHEMBL3904942 | US11155556, No. 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human Aurora kinase B (D25 to A303 residues) expressed in mammalian expression system by Z'LYTE assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50337135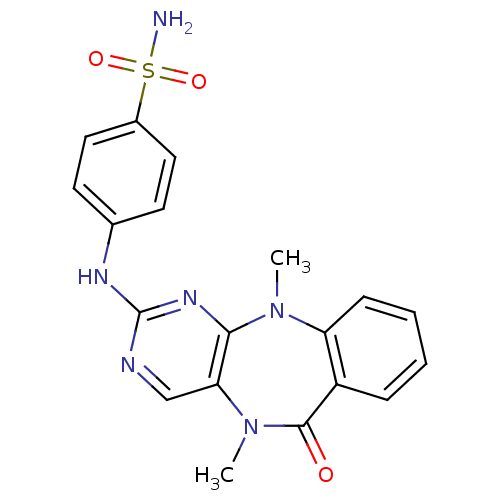 (4-((5,11-dimethyl-6-oxo-6,11-dihydro-5H-benzo[e]py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human Aurora kinase A (E122 to K401 residues) expressed in mammalian expression system by Z'LYTE assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50337135 (4-((5,11-dimethyl-6-oxo-6,11-dihydro-5H-benzo[e]py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50539938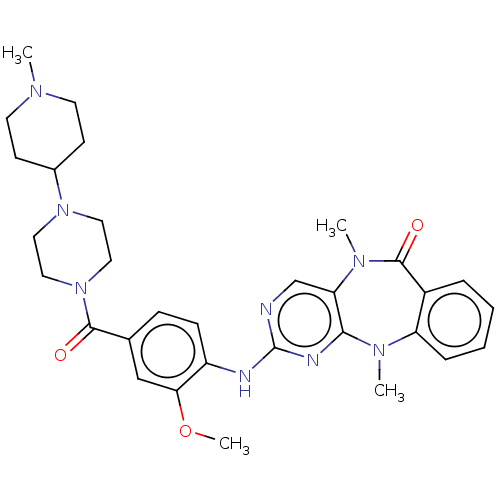 (CHEMBL4647792) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 residues) expressed in baculovirus expression system using LRRKtide as... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50539937 (CHEMBL4636475) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 residues) expressed in baculovirus expression system using LRRKtide as... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50193017 (CHEMBL3904942 | US11155556, No. 17) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 residues) expressed in baculovirus expression system using LRRKtide as... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535570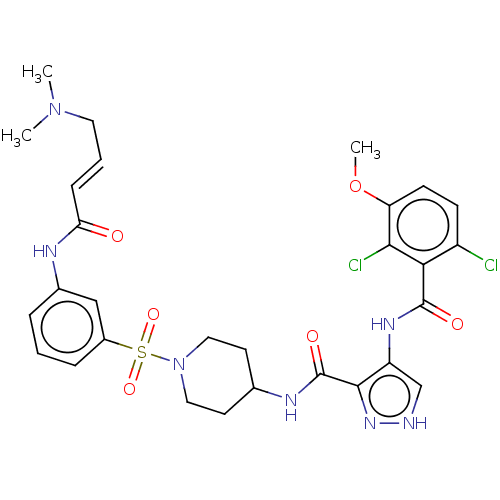 (CHEMBL4579572) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50193031 (CHEMBL3959351 | US11155556, No. 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute Curated by ChEMBL | Assay Description Inhibition of human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system incubated for 60 mins by ADAPTA assay | ACS Med Chem Lett 7: 908-912 (2016) Article DOI: 10.1021/acsmedchemlett.6b00209 BindingDB Entry DOI: 10.7270/Q27P91B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50193031 (CHEMBL3959351 | US11155556, No. 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The enzymatic activities against PI3K-α, PI3K-β, PI3K-γ and PI3K-δ were tested in ADAPTA assays. Activity against AURKB and AURKB... | Citation and Details BindingDB Entry DOI: 10.7270/Q20868G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50337135 (4-((5,11-dimethyl-6-oxo-6,11-dihydro-5H-benzo[e]py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 residues) expressed in baculovirus expression system using LRRKtide as... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50337136 (4-((5,11-dimethyl-6-oxo-6,11-dihydro-5H-benzo[e]py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 residues) expressed in baculovirus expression system using LRRKtide as... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Y (Homo sapiens) | BDBM50535545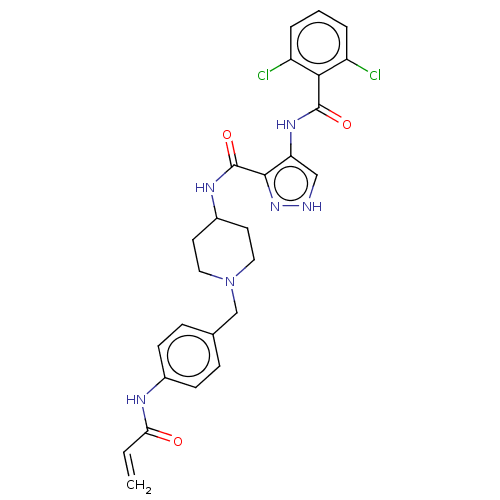 (CHEMBL4519165) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged human full length CDK14/cyclin Y (2 to end residues) preincubated for 30 mins by Lantha screen eu binding assay | Bioorg Med Chem Lett 29: 1985-1993 (2019) Article DOI: 10.1016/j.bmcl.2019.05.024 BindingDB Entry DOI: 10.7270/Q29P355R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50503068 (CHEMBL4483587) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute Curated by ChEMBL | Assay Description Displacement of biotinylated probe from human TAF1 bromodomain 2 (1522 to 1656 residues) expressed in Escherichia coli BL21 (DE3) by alphascreen assa... | ACS Med Chem Lett 10: 1443-1449 (2019) Article DOI: 10.1021/acsmedchemlett.9b00243 BindingDB Entry DOI: 10.7270/Q28P63T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 668 total ) | Next | Last >> |