| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2A |
|---|
| Ligand | BDBM50161646 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1809671 (CHEMBL4309131) |
|---|
| Ki | 112±n/a nM |
|---|
| Citation |  Wild, CT; Miszkiel, JM; Wold, EA; Soto, CA; Ding, C; Hartley, RM; White, MA; Anastasio, NC; Cunningham, KA; Zhou, J Design, Synthesis, and Characterization of 4-Undecylpiperidine-2-carboxamides as Positive Allosteric Modulators of the Serotonin (5-HT) 5-HT J Med Chem62:288-305 (2019) [PubMed] Article Wild, CT; Miszkiel, JM; Wold, EA; Soto, CA; Ding, C; Hartley, RM; White, MA; Anastasio, NC; Cunningham, KA; Zhou, J Design, Synthesis, and Characterization of 4-Undecylpiperidine-2-carboxamides as Positive Allosteric Modulators of the Serotonin (5-HT) 5-HT J Med Chem62:288-305 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2A |
|---|
| Name: | 5-hydroxytryptamine receptor 2A |
|---|
| Synonyms: | 5-HT-2 | 5-HT-2A | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT-2A) | 5-hydroxytryptamine receptor 2A (5HT-2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_HUMAN | HTR2 | HTR2A | Serotonin receptor 2A |
|---|
| Type: | undefined |
|---|
| Mol. Mass.: | 52607.65 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P28223 |
|---|
| Residue: | 471 |
|---|
| Sequence: | MDILCEENTSLSSTTNSLMQLNDDTRLYSNDFNSGEANTSDAFNWTVDSENRTNLSCEGC
LSPSCLSLLHLQEKNWSALLTAVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIAD
MLLGFLVMPVSMLTILYGYRWPLPSKLCAVWIYLDVLFSTASIMHLCAISLDRYVAIQNP
IHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSF
VSFFIPLTIMVITYFLTIKSLQKEATLCVSDLGTRAKLASFSFLPQSSLSSEKLFQRSIH
REPGSYTGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNEDVIGA
LLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENKKPLQLILVNTIPALAYK
SSQLQMGQKKNSKQDAKTTDNDCSMVALGKQHSEEASKDNSDGVNEKVSCV
|
|
|
|---|
| BDBM50161646 |
|---|
| n/a |
|---|
| Name | BDBM50161646 |
|---|
| Synonyms: | (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine | (R)-8-Chloro-1-methyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine | CHEMBL360328 | LORCASERIN |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C11H14ClN |
|---|
| Mol. Mass. | 195.689 |
|---|
| SMILES | C[C@H]1CNCCc2ccc(Cl)cc12 |r| |
|---|
| Structure | 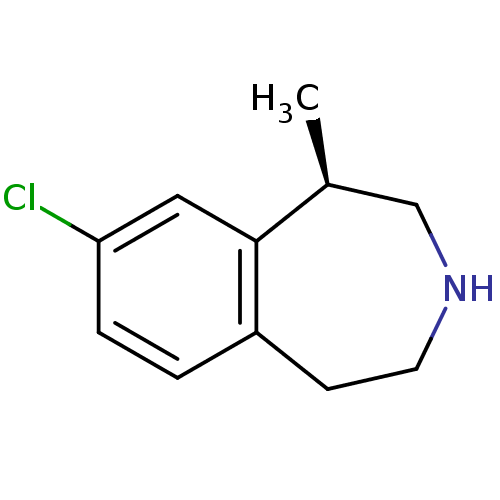
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wild, CT; Miszkiel, JM; Wold, EA; Soto, CA; Ding, C; Hartley, RM; White, MA; Anastasio, NC; Cunningham, KA; Zhou, J Design, Synthesis, and Characterization of 4-Undecylpiperidine-2-carboxamides as Positive Allosteric Modulators of the Serotonin (5-HT) 5-HT J Med Chem62:288-305 (2019) [PubMed] Article
Wild, CT; Miszkiel, JM; Wold, EA; Soto, CA; Ding, C; Hartley, RM; White, MA; Anastasio, NC; Cunningham, KA; Zhou, J Design, Synthesis, and Characterization of 4-Undecylpiperidine-2-carboxamides as Positive Allosteric Modulators of the Serotonin (5-HT) 5-HT J Med Chem62:288-305 (2019) [PubMed] Article