| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50060417 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_51892 (CHEMBL665753) |
|---|
| IC50 | >100000±n/a nM |
|---|
| Citation |  Bromidge, SM; Duckworth, M; Forbes, IT; Ham, P; King, FD; Thewlis, KM; Blaney, FE; Naylor, CB; Blackburn, TP; Kennett, GA; Wood, MD; Clarke, SE 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]- indoline (SB-242084): the first selective and brain penetrant 5-HT2C receptor antagonist. J Med Chem40:3494-6 (1997) [PubMed] Article Bromidge, SM; Duckworth, M; Forbes, IT; Ham, P; King, FD; Thewlis, KM; Blaney, FE; Naylor, CB; Blackburn, TP; Kennett, GA; Wood, MD; Clarke, SE 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]- indoline (SB-242084): the first selective and brain penetrant 5-HT2C receptor antagonist. J Med Chem40:3494-6 (1997) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50060417 |
|---|
| n/a |
|---|
| Name | BDBM50060417 |
|---|
| Synonyms: | 5-Methyl-3,5-dihydro-2H-pyrrolo[2,3-f]indole-1-carboxylic acid pyridin-3-ylamide | CHEMBL297784 | SB 206553 | SB-206553 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H16N4O |
|---|
| Mol. Mass. | 292.3351 |
|---|
| SMILES | Cn1ccc2cc3N(CCc3cc12)C(=O)Nc1cccnc1 |
|---|
| Structure | 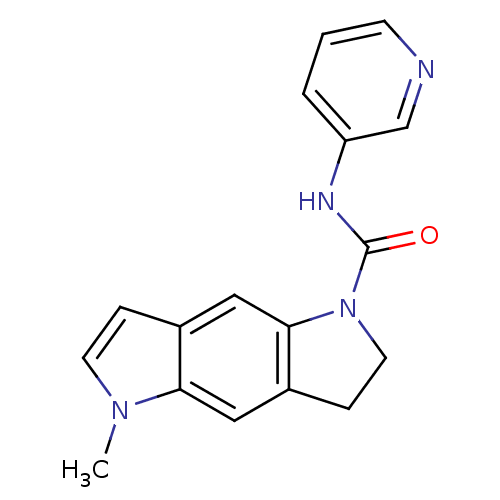
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bromidge, SM; Duckworth, M; Forbes, IT; Ham, P; King, FD; Thewlis, KM; Blaney, FE; Naylor, CB; Blackburn, TP; Kennett, GA; Wood, MD; Clarke, SE 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]- indoline (SB-242084): the first selective and brain penetrant 5-HT2C receptor antagonist. J Med Chem40:3494-6 (1997) [PubMed] Article
Bromidge, SM; Duckworth, M; Forbes, IT; Ham, P; King, FD; Thewlis, KM; Blaney, FE; Naylor, CB; Blackburn, TP; Kennett, GA; Wood, MD; Clarke, SE 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]- indoline (SB-242084): the first selective and brain penetrant 5-HT2C receptor antagonist. J Med Chem40:3494-6 (1997) [PubMed] Article