| Reaction Details |
|---|
 | Report a problem with these data |
| Target | UDP-glucuronosyltransferase 1A1 |
|---|
| Ligand | BDBM50508419 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1833056 (CHEMBL4333064) |
|---|
| IC50 | >50000±n/a nM |
|---|
| Citation |  Lee, D; Pagire, HS; Pagire, SH; Bae, EJ; Dighe, M; Kim, M; Lee, KM; Jang, YK; Jaladi, AK; Jung, KY; Yoo, EK; Gim, HE; Lee, S; Choi, WI; Chi, YI; Song, JS; Bae, MA; Jeon, YH; Lee, GH; Liu, KH; Lee, T; Park, S; Jeon, JH; Lee, IK; Ahn, JH Discovery of Novel Pyruvate Dehydrogenase Kinase 4 Inhibitors for Potential Oral Treatment of Metabolic Diseases. J Med Chem62:575-588 (2019) [PubMed] Article Lee, D; Pagire, HS; Pagire, SH; Bae, EJ; Dighe, M; Kim, M; Lee, KM; Jang, YK; Jaladi, AK; Jung, KY; Yoo, EK; Gim, HE; Lee, S; Choi, WI; Chi, YI; Song, JS; Bae, MA; Jeon, YH; Lee, GH; Liu, KH; Lee, T; Park, S; Jeon, JH; Lee, IK; Ahn, JH Discovery of Novel Pyruvate Dehydrogenase Kinase 4 Inhibitors for Potential Oral Treatment of Metabolic Diseases. J Med Chem62:575-588 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| UDP-glucuronosyltransferase 1A1 |
|---|
| Name: | UDP-glucuronosyltransferase 1A1 |
|---|
| Synonyms: | Bilirubin-specific UDPGT isozyme 1 | GNT1 | UD11_HUMAN | UDP-glucuronosyltransferase 1-1 | UDP-glucuronosyltransferase 1-A | UDP-glucuronosyltransferase 1A1 | UDPGT 1-1 | UGT-1A | UGT1 | UGT1*1 | UGT1-01 | UGT1.1 | UGT1A | UGT1A1 | Uridine-5'-diphosphoglucuronosyltransferase 1A1 | hUG-BR1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 59604.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P22309 |
|---|
| Residue: | 533 |
|---|
| Sequence: | MAVESQGGRPLVLGLLLCVLGPVVSHAGKILLIPVDGSHWLSMLGAIQQLQQRGHEIVVL
APDASLYIRDGAFYTLKTYPVPFQREDVKESFVSLGHNVFENDSFLQRVIKTYKKIKKDS
AMLLSGCSHLLHNKELMASLAESSFDVMLTDPFLPCSPIVAQYLSLPTVFFLHALPCSLE
FEATQCPNPFSYVPRPLSSHSDHMTFLQRVKNMLIAFSQNFLCDVVYSPYATLASEFLQR
EVTVQDLLSSASVWLFRSDFVKDYPRPIMPNMVFVGGINCLHQNPLSQEFEAYINASGEH
GIVVFSLGSMVSEIPEKKAMAIADALGKIPQTVLWRYTGTRPSNLANNTILVKWLPQNDL
LGHPMTRAFITHAGSHGVYESICNGVPMVMMPLFGDQMDNAKRMETKGAGVTLNVLEMTS
EDLENALKAVINDKSYKENIMRLSSLHKDRPVEPLDLAVFWVEFVMRHKGAPHLRPAAHD
LTWYQYHSLDVIGFLLAVVLTVAFITFKCCAYGYRKCLGKKGRVKKAHKSKTH
|
|
|
|---|
| BDBM50508419 |
|---|
| n/a |
|---|
| Name | BDBM50508419 |
|---|
| Synonyms: | CHEMBL4471264 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H20ClN3O2 |
|---|
| Mol. Mass. | 393.866 |
|---|
| SMILES | Cl.O=C1c2ccccc2C(=O)c2c(cccc12)-c1cnn(c1)C1CCNCC1 |
|---|
| Structure | 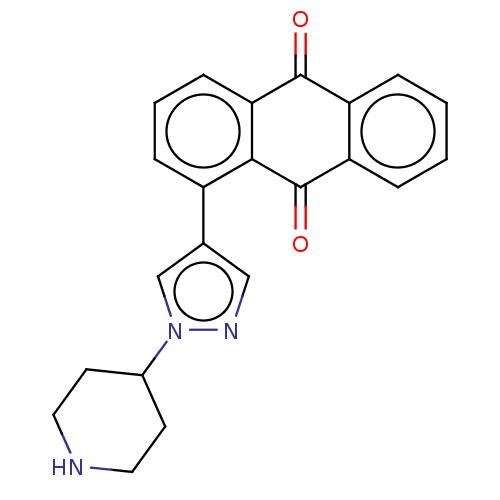
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lee, D; Pagire, HS; Pagire, SH; Bae, EJ; Dighe, M; Kim, M; Lee, KM; Jang, YK; Jaladi, AK; Jung, KY; Yoo, EK; Gim, HE; Lee, S; Choi, WI; Chi, YI; Song, JS; Bae, MA; Jeon, YH; Lee, GH; Liu, KH; Lee, T; Park, S; Jeon, JH; Lee, IK; Ahn, JH Discovery of Novel Pyruvate Dehydrogenase Kinase 4 Inhibitors for Potential Oral Treatment of Metabolic Diseases. J Med Chem62:575-588 (2019) [PubMed] Article
Lee, D; Pagire, HS; Pagire, SH; Bae, EJ; Dighe, M; Kim, M; Lee, KM; Jang, YK; Jaladi, AK; Jung, KY; Yoo, EK; Gim, HE; Lee, S; Choi, WI; Chi, YI; Song, JS; Bae, MA; Jeon, YH; Lee, GH; Liu, KH; Lee, T; Park, S; Jeon, JH; Lee, IK; Ahn, JH Discovery of Novel Pyruvate Dehydrogenase Kinase 4 Inhibitors for Potential Oral Treatment of Metabolic Diseases. J Med Chem62:575-588 (2019) [PubMed] Article