| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Beta-lactamase |
|---|
| Ligand | BDBM26140 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_35528 (CHEMBL649554) |
|---|
| Ki | 220±n/a nM |
|---|
| Citation |  Weston, GS; Blázquez, J; Baquero, F; Shoichet, BK Structure-based enhancement of boronic acid-based inhibitors of AmpC beta-lactamase. J Med Chem41:4577-86 (1998) [PubMed] Article Weston, GS; Blázquez, J; Baquero, F; Shoichet, BK Structure-based enhancement of boronic acid-based inhibitors of AmpC beta-lactamase. J Med Chem41:4577-86 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Beta-lactamase |
|---|
| Name: | Beta-lactamase |
|---|
| Synonyms: | AMPC_ECOLI | Beta-lactamase | Beta-lactamase (AmpC) | Beta-lactamase AmpC | Cephalosporinase | Escherichia coli K-12 | ampA | ampC |
|---|
| Type: | Protien |
|---|
| Mol. Mass.: | 41561.62 |
|---|
| Organism: | Escherichia coli |
|---|
| Description: | P00811 |
|---|
| Residue: | 377 |
|---|
| Sequence: | MFKTTLCALLITASCSTFAAPQQINDIVHRTITPLIEQQKIPGMAVAVIYQGKPYYFTWG
YADIAKKQPVTQQTLFELGSVSKTFTGVLGGDAIARGEIKLSDPTTKYWPELTAKQWNGI
TLLHLATYTAGGLPLQVPDEVKSSSDLLRFYQNWQPAWAPGTQRLYANSSIGLFGALAVK
PSGLSFEQAMQTRVFQPLKLNHTWINVPPAEEKNYAWGYREGKAVHVSPGALDAEAYGVK
STIEDMARWVQSNLKPLDINEKTLQQGIQLAQSRYWQTGDMYQGLGWEMLDWPVNPDSII
NGSDNKIALAARPVKAITPPTPAVRASWVHKTGATGGFGSYVAFIPEKELGIVMLANKNY
PNPARVDAAWQILNALQ
|
|
|
|---|
| BDBM26140 |
|---|
| n/a |
|---|
| Name | BDBM26140 |
|---|
| Synonyms: | 1-benzofuran-2-ylboranediol | 1-benzofuran-2-ylboronic acid, 19 | CHEMBL143399 | Phenylboronic acid, 21 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C8H7BO3 |
|---|
| Mol. Mass. | 161.95 |
|---|
| SMILES | OB(O)c1cc2ccccc2o1 |
|---|
| Structure | 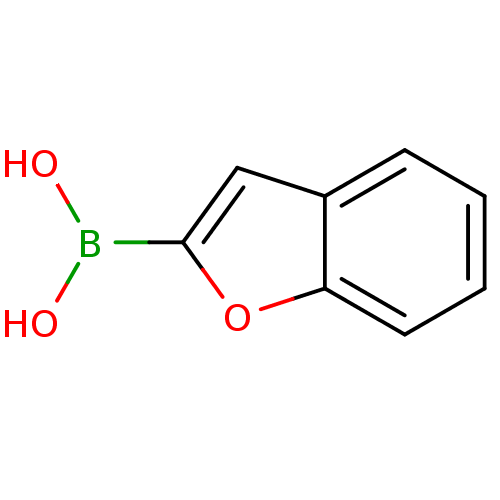
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Weston, GS; Blázquez, J; Baquero, F; Shoichet, BK Structure-based enhancement of boronic acid-based inhibitors of AmpC beta-lactamase. J Med Chem41:4577-86 (1998) [PubMed] Article
Weston, GS; Blázquez, J; Baquero, F; Shoichet, BK Structure-based enhancement of boronic acid-based inhibitors of AmpC beta-lactamase. J Med Chem41:4577-86 (1998) [PubMed] Article