

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50281817 (CHEMBL1712377) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human HRH1 expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 6830-6845 (2018) Article DOI: 10.1021/acs.jmedchem.8b00718 BindingDB Entry DOI: 10.7270/Q2BG2RJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579543 (US11484525, Compound DD-144-ANTAGONIST-Covalent) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nAChR expressed in human recombinant SH-SY5Y cell membranes after 120 mins | J Med Chem 60: 9239-9250 (2017) Article DOI: 10.1021/acs.jmedchem.7b01113 BindingDB Entry DOI: 10.7270/Q2445PW2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585913 (CHEMBL5093295) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50246607 (CHEMBL4083241) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nAChR expressed in human recombinant SH-SY5Y cell membranes after 120 mins | J Med Chem 60: 9239-9250 (2017) Article DOI: 10.1021/acs.jmedchem.7b01113 BindingDB Entry DOI: 10.7270/Q2445PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50251208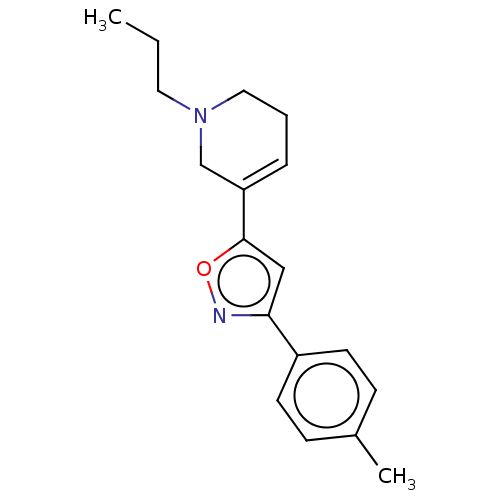 (CHEMBL4088272) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM26113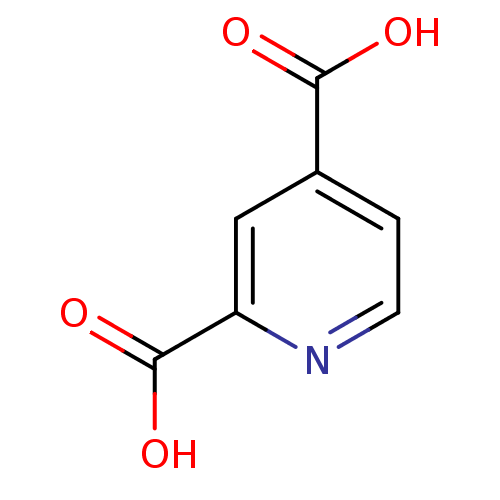 (2,4 PDCA | cid_10365 | pyridine carboxylate, 6a | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using A... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585914 (CHEMBL5079273) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 1 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50048866 (1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585912 (CHEMBL5075486) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579540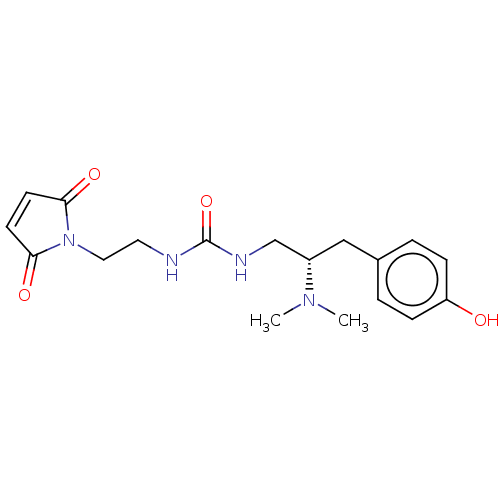 (US11484525, Compound DD-120-D1-covalent-compound) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579486 (US11484525, Compound Naloxone) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585909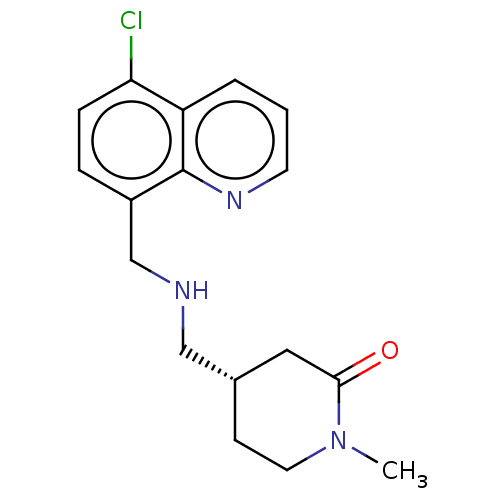 (CHEMBL5089996) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579486 (US11484525, Compound Naloxone) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579538 (US11484525, Compound BD-131LR) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579515 (US11484525, Compound DD-88B-S) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579541 (US11484525, Compound DD-138) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50448377 (CHEMBL3121473) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl-scopolamine bromide from human recombinant muscarinic M2 receptor expressed in HEK293T cell membranes after 1 hr by liqu... | J Med Chem 60: 9239-9250 (2017) Article DOI: 10.1021/acs.jmedchem.7b01113 BindingDB Entry DOI: 10.7270/Q2445PW2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579544 (US11484525, Compound DD-158) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM462406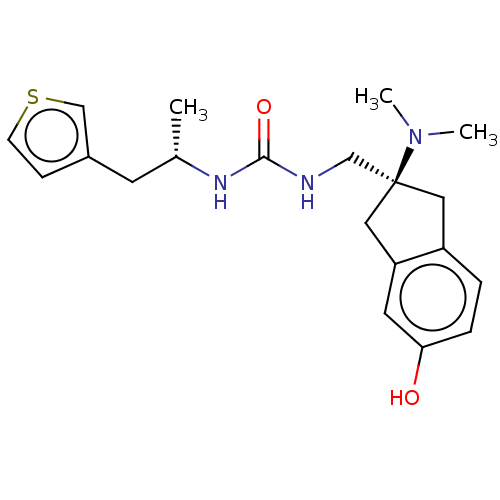 (US10780078, Compound DD 297A) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description This study supports a structure-based approach for GPCR ligand discovery. These new chemotypes may stabilize receptor conformations not explored prev... | Bioorg Med Chem Lett 5: 2707-12 (1995) Article DOI: 10.1016/0960-894X(95)00461-2 BindingDB Entry DOI: 10.7270/Q2XW4GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579542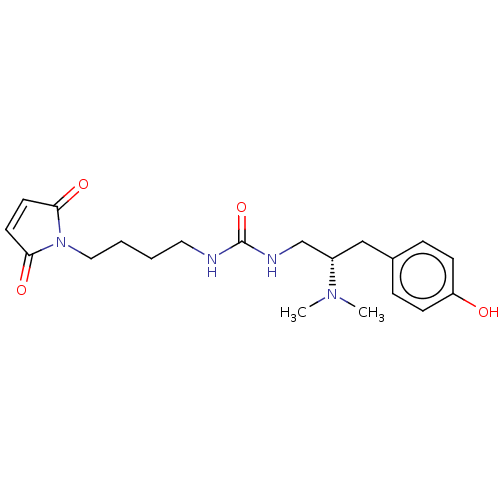 (US11484525, Compound DD-139) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579537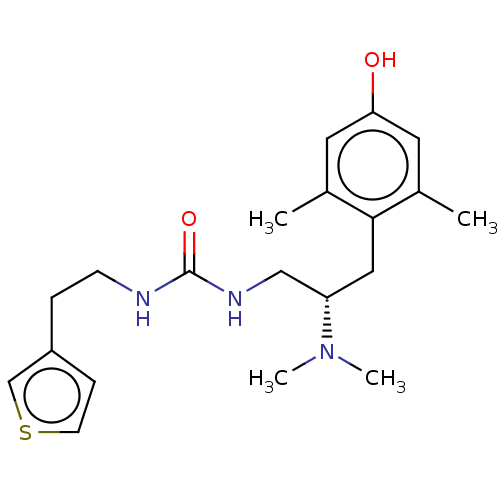 (US11484525, Compound DD-34L-ANTAGONIST) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM462406 (US10780078, Compound DD 297A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article US Patent | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description This study supports a structure-based approach for GPCR ligand discovery. These new chemotypes may stabilize receptor conformations not explored prev... | Bioorg Med Chem Lett 5: 2707-12 (1995) Article DOI: 10.1016/0960-894X(95)00461-2 BindingDB Entry DOI: 10.7270/Q2XW4GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579539 (US11484525, Compound BD-131LS) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585910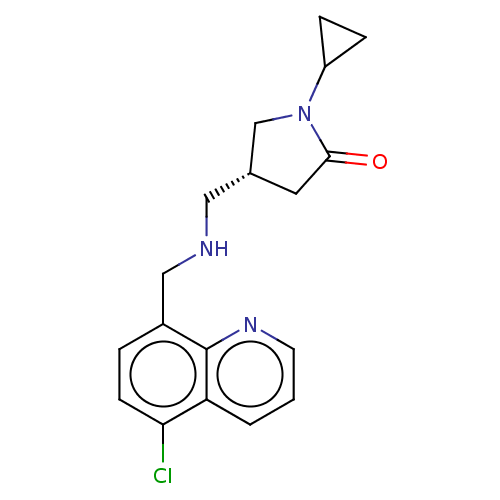 (CHEMBL5094012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585911 (CHEMBL5093969) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579527 (US11484525, Compound DD-57L) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579522 (US11484525, Compound DD-63L-LB-S) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocin from the Sigma1 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50225373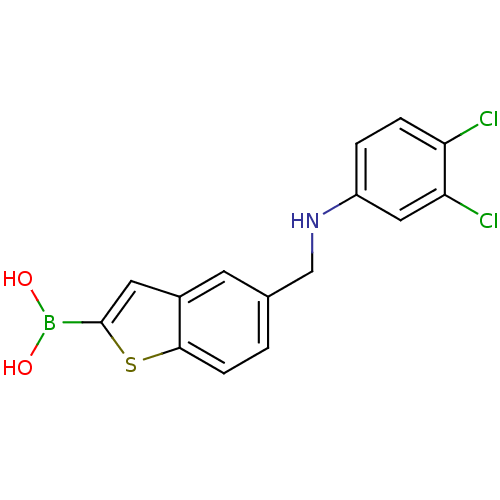 (CHEMBL396872 | pinacol 5-[(3,4-dichlorophenylamino...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Modena e Reggio Emilia Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-lactamase | J Med Chem 50: 5644-54 (2007) Article DOI: 10.1021/jm070643q BindingDB Entry DOI: 10.7270/Q21N80W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579539 (US11484525, Compound BD-131LS) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579538 (US11484525, Compound BD-131LR) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579520 (US11484525, Compound DD-63L-LA-R | US11484525, Com...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM579538 (US11484525, Compound BD-131LR) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50518036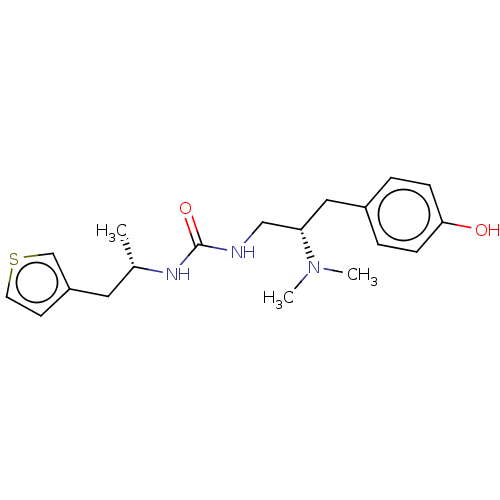 (CHEMBL4467777 | US11484525, Compound BD-122LS-PZM2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579543 (US11484525, Compound DD-144-ANTAGONIST-Covalent) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579520 (US11484525, Compound DD-63L-LA-R | US11484525, Com...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50281507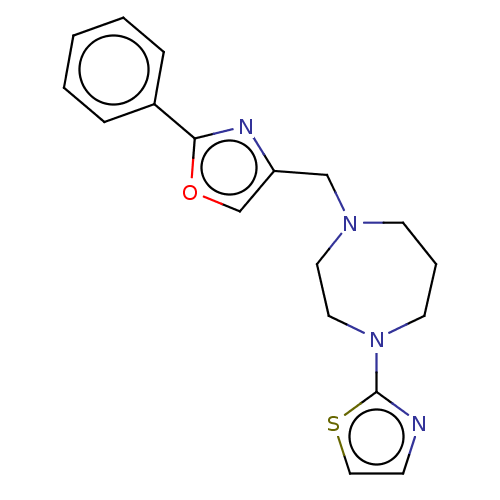 (CHEMBL4161436) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human HRH1 expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 61: 6830-6845 (2018) Article DOI: 10.1021/acs.jmedchem.8b00718 BindingDB Entry DOI: 10.7270/Q2BG2RJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50115618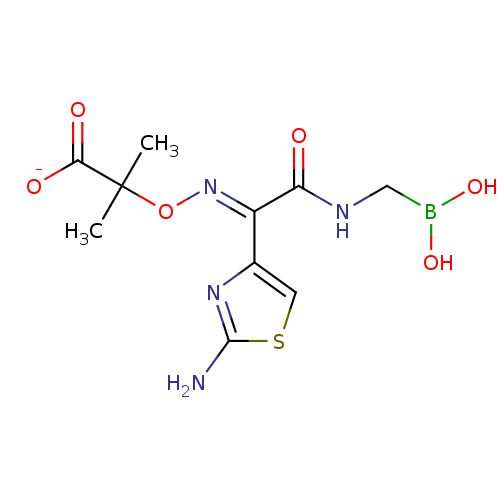 (2-[(2-Amino-thiazol-4-yl)-(dihydroxyboranylmethyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Binding affinity of the compound towards AmpC beta-lactamase binding site from Escherichia coli | J Med Chem 45: 3222-34 (2002) BindingDB Entry DOI: 10.7270/Q2CN737M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Activity of compound against Muscarinic acetylcholine receptor M1 (CHRM1) by displacement of 3H-QNB | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM39816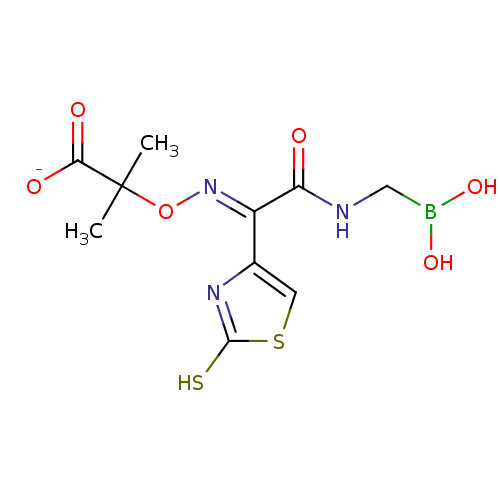 (Acylglycineboronic acid, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Northwestern University | Assay Description Enzyme inhibition assay using AmpC or TEM-1 from escherichia coli. | Chem Biol 8: 17-31 (2001) Article DOI: 10.1016/S1074-5521(00)00052-1 BindingDB Entry DOI: 10.7270/Q2CC0Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579522 (US11484525, Compound DD-63L-LB-S) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579557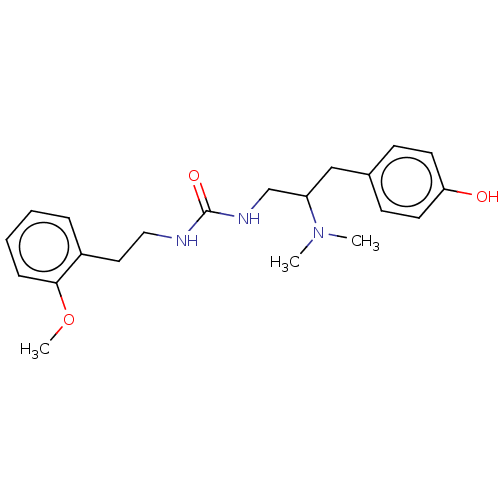 (US11484525, Compound DD198) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM579486 (US11484525, Compound Naloxone) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50246733 (CHEMBL4100938) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl-scopolamine bromide from human recombinant muscarinic M1 receptor expressed in HEK293T cell membranes after 1 hr by liqu... | J Med Chem 60: 9239-9250 (2017) Article DOI: 10.1021/acs.jmedchem.7b01113 BindingDB Entry DOI: 10.7270/Q2445PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM579533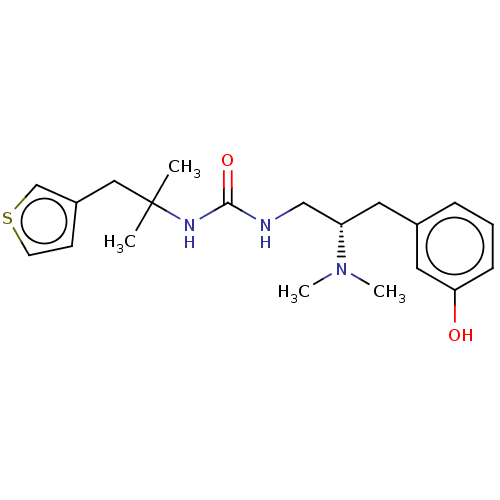 (US11484525, Compound DD-46L) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor [N127C] (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | US Patent | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Each compound was initially tested at 20 μM and was incubated with 3H-DPN at a concentration equal to the Kd (0.4 nM) of the radioligand in _... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ14V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50253157 ((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG) Curated by ChEMBL | Assay Description Displacement of [3H]-DTG from the Sigma2 receptor | Nature 583: 459-468 (2020) Article DOI: 10.1038/s41586-020-2286-9 BindingDB Entry DOI: 10.7270/Q29Z984K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM26139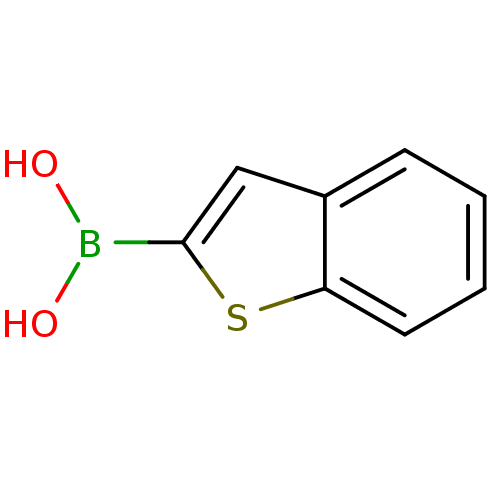 (1-benzothiophen-2-ylboranediol | 1-benzothiophen-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Medical School Curated by ChEMBL | Assay Description Inhibitory activity against E. coli AmpC beta-lactamase. | J Med Chem 41: 4577-86 (1998) Article DOI: 10.1021/jm980343w BindingDB Entry DOI: 10.7270/Q22N51FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 2095 total ) | Next | Last >> |