| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone acetyltransferase KAT2A |
|---|
| Ligand | BDBM50512964 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1850999 (CHEMBL4351623) |
|---|
| Kd | 105±n/a nM |
|---|
| Citation |  Huang, L; Li, H; Li, L; Niu, L; Seupel, R; Wu, C; Cheng, W; Chen, C; Ding, B; Brennan, PE; Yang, S Discovery of Pyrrolo[3,2- d]pyrimidin-4-one Derivatives as a New Class of Potent and Cell-Active Inhibitors of P300/CBP-Associated Factor Bromodomain. J Med Chem62:4526-4542 (2019) [PubMed] Article Huang, L; Li, H; Li, L; Niu, L; Seupel, R; Wu, C; Cheng, W; Chen, C; Ding, B; Brennan, PE; Yang, S Discovery of Pyrrolo[3,2- d]pyrimidin-4-one Derivatives as a New Class of Potent and Cell-Active Inhibitors of P300/CBP-Associated Factor Bromodomain. J Med Chem62:4526-4542 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone acetyltransferase KAT2A |
|---|
| Name: | Histone acetyltransferase KAT2A |
|---|
| Synonyms: | GCN5 | GCN5 | GCN5L2 | General control of amino acid synthesis protein 5-like 2 | HGCN5 | Histone acetyltransferase GCN5 | Histone acetyltransferase KAT2A | Histone acetyltransferase KAT2A/KAT2B | HsGCN5 | KAT2A | KAT2A_HUMAN | Lysine acetyltransferase 2A | STAF97 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 93956.22 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_100876 |
|---|
| Residue: | 837 |
|---|
| Sequence: | MAEPSQAPTPAPAAQPRPLQSPAPAPTPTPAPSPASAPIPTPTPAPAPAPAAAPAGSTGT
GGPGVGSGGAGSGGDPARPGLSQQQRASQRKAQVRGLPRAKKLEKLGVFSACKANETCKC
NGWKNPKPPTAPRMDLQQPAANLSELCRSCEHPLADHVSHLENVSEDEINRLLGMVVDVE
NLFMSVHKEEDTDTKQVYFYLFKLLRKCILQMTRPVVEGSLGSPPFEKPNIEQGVLNFVQ
YKFSHLAPRERQTMFELSKMFLLCLNYWKLETPAQFRQRSQAEDVATYKVNYTRWLCYCH
VPQSCDSLPRYETTHVFGRSLLRSIFTVTRRQLLEKFRVEKDKLVPEKRTLILTHFPKFL
SMLEEEIYGANSPIWESGFTMPPSEGTQLVPRPASVSAAVVPSTPIFSPSMGGGSNSSLS
LDSAGAEPMPGEKRTLPENLTLEDAKRLRVMGDIPMELVNEVMLTITDPAAMLGPETSLL
SANAARDETARLEERRGIIEFHVIGNSLTPKANRRVLLWLVGLQNVFSHQLPRMPKEYIA
RLVFDPKHKTLALIKDGRVIGGICFRMFPTQGFTEIVFCAVTSNEQVKGYGTHLMNHLKE
YHIKHNILYFLTYADEYAIGYFKKQGFSKDIKVPKSRYLGYIKDYEGATLMECELNPRIP
YTELSHIIKKQKEIIKKLIERKQAQIRKVYPGLSCFKEGVRQIPVESVPGIRETGWKPLG
KEKGKELKDPDQLYTTLKNLLAQIKSHPSAWPFMEPVKKSEAPDYYEVIRFPIDLKTMTE
RLRSRYYVTRKLFVADLQRVIANCREYNPPDSEYCRCASALEKFFYFKLKEGGLIDK
|
|
|
|---|
| BDBM50512964 |
|---|
| n/a |
|---|
| Name | BDBM50512964 |
|---|
| Synonyms: | CHEMBL4574669 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H25N5O3 |
|---|
| Mol. Mass. | 395.4549 |
|---|
| SMILES | CN1C[C@@H](C[C@@H](C1)c1ccc2OCCOc2c1)Nc1nc2cc[nH]c2c(=O)n1C |r| |
|---|
| Structure | 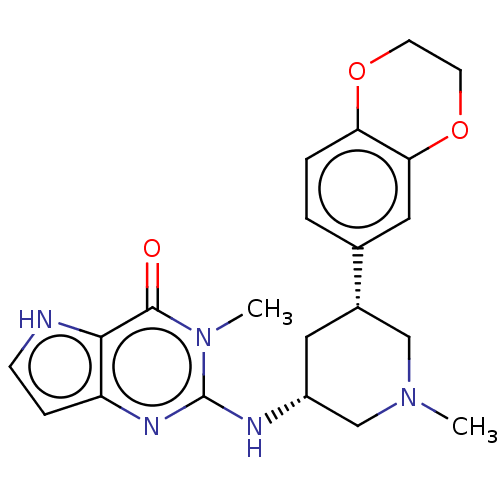
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Huang, L; Li, H; Li, L; Niu, L; Seupel, R; Wu, C; Cheng, W; Chen, C; Ding, B; Brennan, PE; Yang, S Discovery of Pyrrolo[3,2- d]pyrimidin-4-one Derivatives as a New Class of Potent and Cell-Active Inhibitors of P300/CBP-Associated Factor Bromodomain. J Med Chem62:4526-4542 (2019) [PubMed] Article
Huang, L; Li, H; Li, L; Niu, L; Seupel, R; Wu, C; Cheng, W; Chen, C; Ding, B; Brennan, PE; Yang, S Discovery of Pyrrolo[3,2- d]pyrimidin-4-one Derivatives as a New Class of Potent and Cell-Active Inhibitors of P300/CBP-Associated Factor Bromodomain. J Med Chem62:4526-4542 (2019) [PubMed] Article