 Found 2014 hits with Last Name = 'brennan' and Initial = 'pe'
Found 2014 hits with Last Name = 'brennan' and Initial = 'pe' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50513361
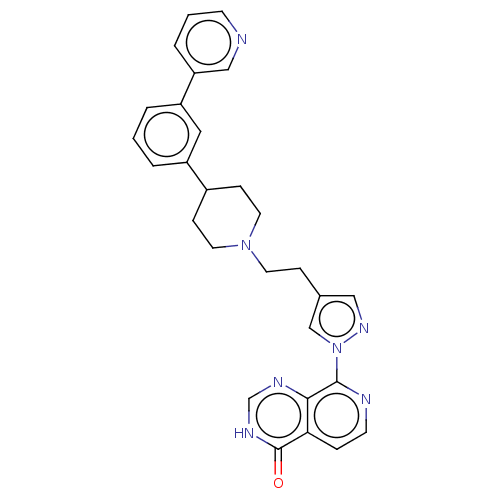
(CHEMBL4567766)Show SMILES O=c1[nH]cnc2c(nccc12)-n1cc(CCN2CCC(CC2)c2cccc(c2)-c2cccnc2)cn1 Show InChI InChI=1S/C28H27N7O/c36-28-25-6-11-30-27(26(25)31-19-32-28)35-18-20(16-33-35)7-12-34-13-8-21(9-14-34)22-3-1-4-23(15-22)24-5-2-10-29-17-24/h1-6,10-11,15-19,21H,7-9,12-14H2,(H,31,32,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM5B (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50513345
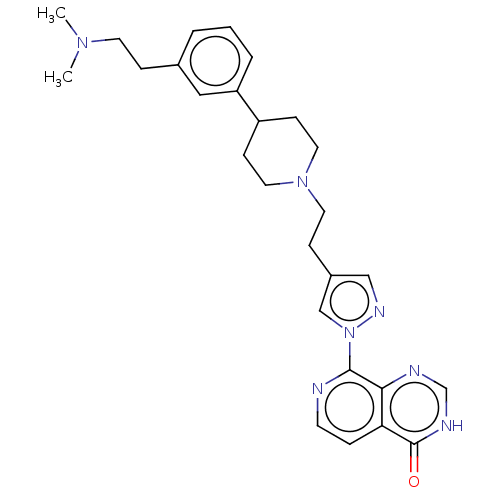
(CHEMBL4438830)Show SMILES CN(C)CCc1cccc(c1)C1CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1 Show InChI InChI=1S/C27H33N7O/c1-32(2)12-7-20-4-3-5-23(16-20)22-9-14-33(15-10-22)13-8-21-17-31-34(18-21)26-25-24(6-11-28-26)27(35)30-19-29-25/h3-6,11,16-19,22H,7-10,12-15H2,1-2H3,(H,29,30,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM5B (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50151923
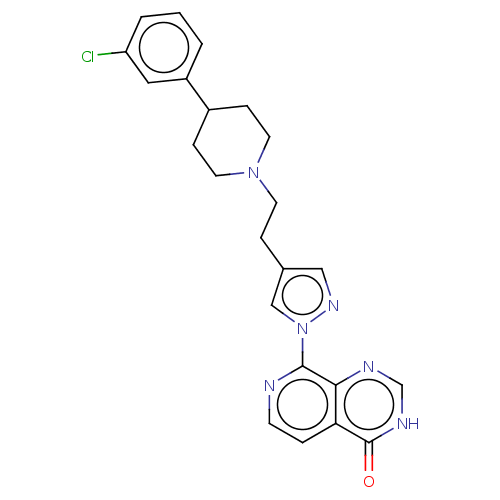
(CHEMBL3774537)Show SMILES Clc1cccc(c1)C1CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1 Show InChI InChI=1S/C23H23ClN6O/c24-19-3-1-2-18(12-19)17-6-10-29(11-7-17)9-5-16-13-28-30(14-16)22-21-20(4-8-25-22)23(31)27-15-26-21/h1-4,8,12-15,17H,5-7,9-11H2,(H,26,27,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM5B (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50513360
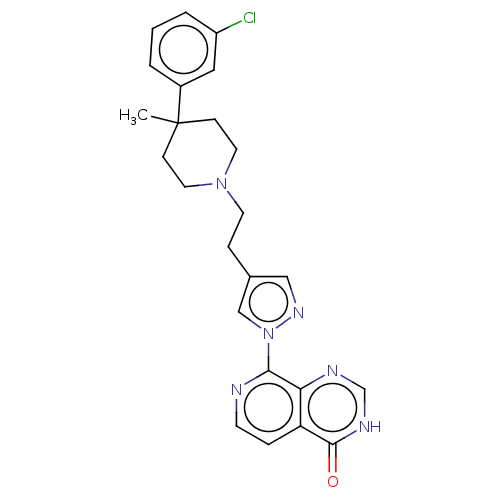
(CHEMBL4573390)Show SMILES CC1(CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C24H25ClN6O/c1-24(18-3-2-4-19(25)13-18)7-11-30(12-8-24)10-6-17-14-29-31(15-17)22-21-20(5-9-26-22)23(32)28-16-27-21/h2-5,9,13-16H,6-8,10-12H2,1H3,(H,27,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM5B (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50513345

(CHEMBL4438830)Show SMILES CN(C)CCc1cccc(c1)C1CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1 Show InChI InChI=1S/C27H33N7O/c1-32(2)12-7-20-4-3-5-23(16-20)22-9-14-33(15-10-22)13-8-21-17-31-34(18-21)26-25-24(6-11-28-26)27(35)30-19-29-25/h3-6,11,16-19,22H,7-10,12-15H2,1-2H3,(H,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM4A (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50151923

(CHEMBL3774537)Show SMILES Clc1cccc(c1)C1CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1 Show InChI InChI=1S/C23H23ClN6O/c24-19-3-1-2-18(12-19)17-6-10-29(11-7-17)9-5-16-13-28-30(14-16)22-21-20(4-8-25-22)23(31)27-15-26-21/h1-4,8,12-15,17H,5-7,9-11H2,(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM4A (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50513361

(CHEMBL4567766)Show SMILES O=c1[nH]cnc2c(nccc12)-n1cc(CCN2CCC(CC2)c2cccc(c2)-c2cccnc2)cn1 Show InChI InChI=1S/C28H27N7O/c36-28-25-6-11-30-27(26(25)31-19-32-28)35-18-20(16-33-35)7-12-34-13-8-21(9-14-34)22-3-1-4-23(15-22)24-5-2-10-29-17-24/h1-6,10-11,15-19,21H,7-9,12-14H2,(H,31,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM4A (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50513344
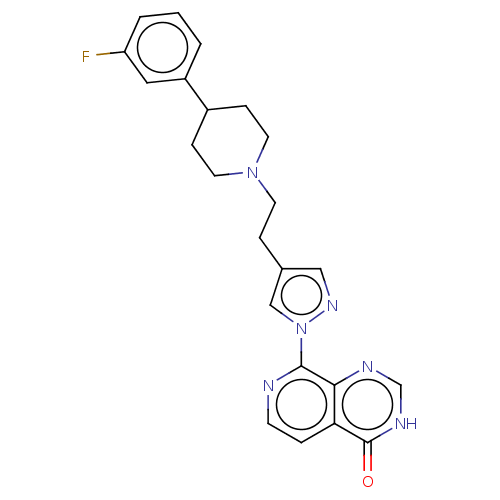
(CHEMBL4447515)Show SMILES Fc1cccc(c1)C1CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1 Show InChI InChI=1S/C23H23FN6O/c24-19-3-1-2-18(12-19)17-6-10-29(11-7-17)9-5-16-13-28-30(14-16)22-21-20(4-8-25-22)23(31)27-15-26-21/h1-4,8,12-15,17H,5-7,9-11H2,(H,26,27,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM5B (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50513348
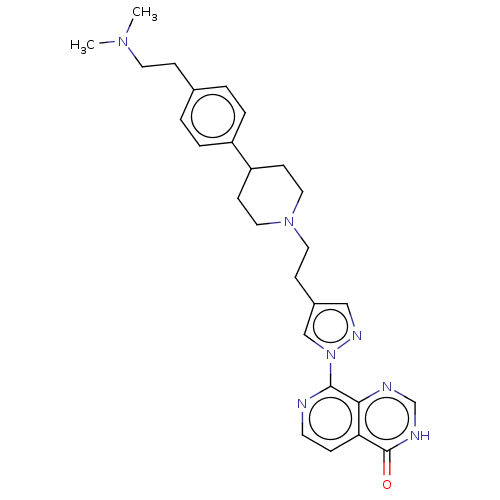
(CHEMBL4585876)Show SMILES CN(C)CCc1ccc(cc1)C1CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1 Show InChI InChI=1S/C27H33N7O/c1-32(2)13-8-20-3-5-22(6-4-20)23-10-15-33(16-11-23)14-9-21-17-31-34(18-21)26-25-24(7-12-28-26)27(35)30-19-29-25/h3-7,12,17-19,23H,8-11,13-16H2,1-2H3,(H,29,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM4A (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50513344

(CHEMBL4447515)Show SMILES Fc1cccc(c1)C1CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1 Show InChI InChI=1S/C23H23FN6O/c24-19-3-1-2-18(12-19)17-6-10-29(11-7-17)9-5-16-13-28-30(14-16)22-21-20(4-8-25-22)23(31)27-15-26-21/h1-4,8,12-15,17H,5-7,9-11H2,(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM4A (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50513343
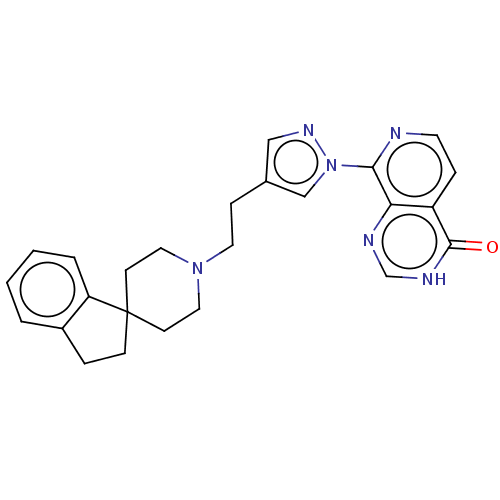
(CHEMBL4449500)Show SMILES O=c1[nH]cnc2c(nccc12)-n1cc(CCN2CCC3(CCc4ccccc34)CC2)cn1 Show InChI InChI=1S/C25H26N6O/c32-24-20-6-11-26-23(22(20)27-17-28-24)31-16-18(15-29-31)7-12-30-13-9-25(10-14-30)8-5-19-3-1-2-4-21(19)25/h1-4,6,11,15-17H,5,7-10,12-14H2,(H,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM4A (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50513348

(CHEMBL4585876)Show SMILES CN(C)CCc1ccc(cc1)C1CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1 Show InChI InChI=1S/C27H33N7O/c1-32(2)13-8-20-3-5-22(6-4-20)23-10-15-33(16-11-23)14-9-21-17-31-34(18-21)26-25-24(7-12-28-26)27(35)30-19-29-25/h3-7,12,17-19,23H,8-11,13-16H2,1-2H3,(H,29,30,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM5B (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50513360

(CHEMBL4573390)Show SMILES CC1(CCN(CCc2cnn(c2)-c2nccc3c2nc[nH]c3=O)CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C24H25ClN6O/c1-24(18-3-2-4-19(25)13-18)7-11-30(12-8-24)10-6-17-14-29-31(15-17)22-21-20(5-9-26-22)23(32)28-16-27-21/h2-5,9,13-16H,6-8,10-12H2,1H3,(H,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM4A (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50513343

(CHEMBL4449500)Show SMILES O=c1[nH]cnc2c(nccc12)-n1cc(CCN2CCC3(CCc4ccccc34)CC2)cn1 Show InChI InChI=1S/C25H26N6O/c32-24-20-6-11-26-23(22(20)27-17-28-24)31-16-18(15-29-31)7-12-30-13-9-25(10-14-30)8-5-19-3-1-2-4-21(19)25/h1-4,6,11,15-17H,5,7-10,12-14H2,(H,27,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM5B (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019696
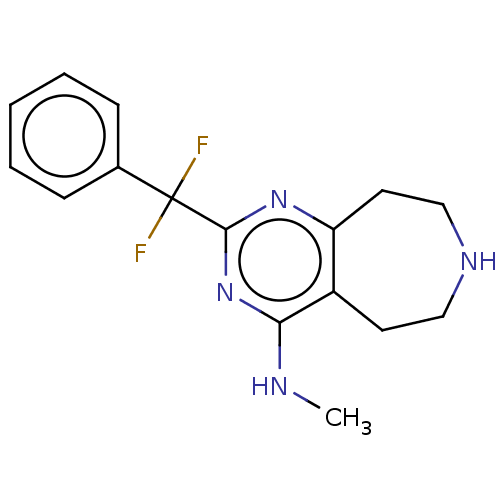
(CHEMBL3286557)Show InChI InChI=1S/C16H18F2N4/c1-19-14-12-7-9-20-10-8-13(12)21-15(22-14)16(17,18)11-5-3-2-4-6-11/h2-6,20H,7-10H2,1H3,(H,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50161646
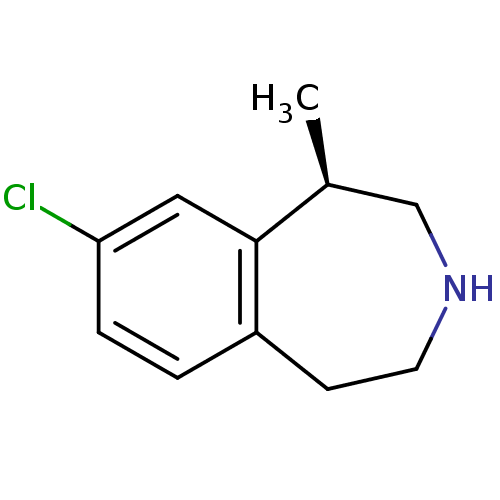
((1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-ben...)Show InChI InChI=1S/C11H14ClN/c1-8-7-13-5-4-9-2-3-10(12)6-11(8)9/h2-3,6,8,13H,4-5,7H2,1H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2C receptor expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem Lett 21: 2715-20 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.120
BindingDB Entry DOI: 10.7270/Q2HD7VZZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
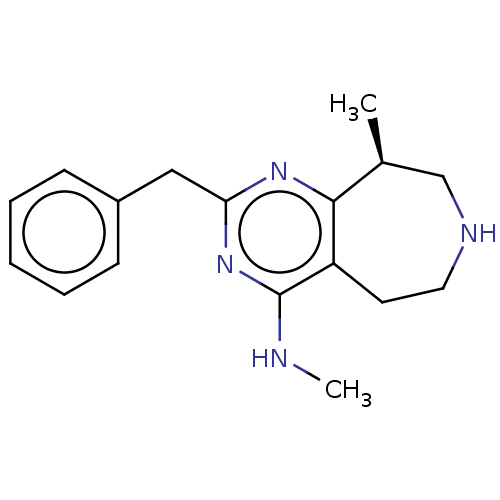
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019685
(CHEMBL3286556)Show InChI InChI=1S/C17H22N4/c1-12-11-19-9-8-14-16(12)20-15(21-17(14)18-2)10-13-6-4-3-5-7-13/h3-7,12,19H,8-11H2,1-2H3,(H,18,20,21)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50513346
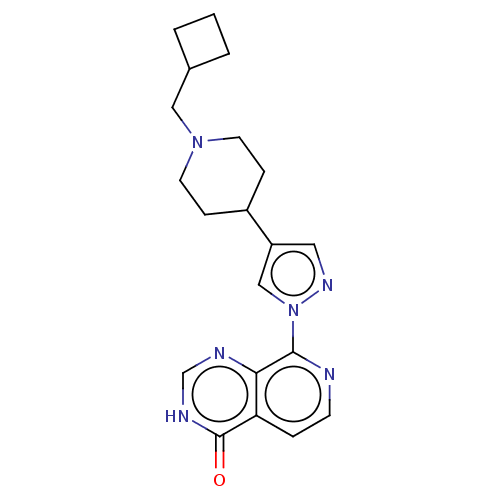
(CHEMBL4525269)Show SMILES O=c1[nH]cnc2c(nccc12)-n1cc(cn1)C1CCN(CC2CCC2)CC1 Show InChI InChI=1S/C20H24N6O/c27-20-17-4-7-21-19(18(17)22-13-23-20)26-12-16(10-24-26)15-5-8-25(9-6-15)11-14-2-1-3-14/h4,7,10,12-15H,1-3,5-6,8-9,11H2,(H,22,23,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM5B (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019682
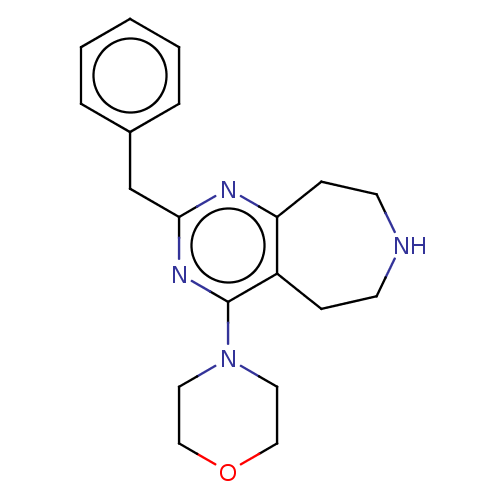
(CHEMBL3286564)Show InChI InChI=1S/C19H24N4O/c1-2-4-15(5-3-1)14-18-21-17-7-9-20-8-6-16(17)19(22-18)23-10-12-24-13-11-23/h1-5,20H,6-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50513346

(CHEMBL4525269)Show SMILES O=c1[nH]cnc2c(nccc12)-n1cc(cn1)C1CCN(CC2CCC2)CC1 Show InChI InChI=1S/C20H24N6O/c27-20-17-4-7-21-19(18(17)22-13-23-20)26-12-16(10-24-26)15-5-8-25(9-6-15)11-14-2-1-3-14/h4,7,10,12-15H,1-3,5-6,8-9,11H2,(H,22,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to KDM4A (unknown origin) |
Eur J Med Chem 177: 316-337 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.041
BindingDB Entry DOI: 10.7270/Q25B05TF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019664

(CHEMBL3286562)Show InChI InChI=1S/C17H22N4/c1-2-19-17-14-8-10-18-11-9-15(14)20-16(21-17)12-13-6-4-3-5-7-13/h3-7,18H,2,8-12H2,1H3,(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019691
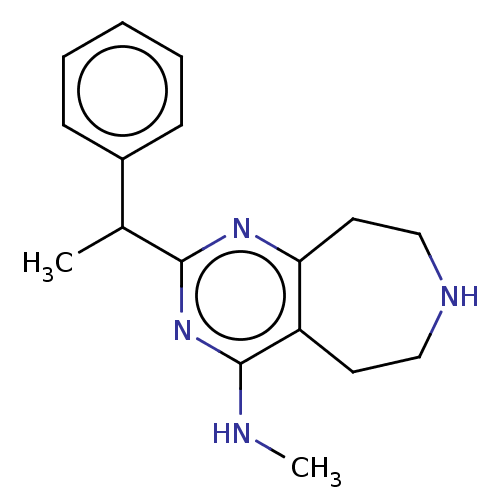
(CHEMBL3286565)Show InChI InChI=1S/C17H22N4/c1-12(13-6-4-3-5-7-13)16-20-15-9-11-19-10-8-14(15)17(18-2)21-16/h3-7,12,19H,8-11H2,1-2H3,(H,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019663

(CHEMBL3286561)Show InChI InChI=1S/C16H20N4/c1-17-16-13-7-9-18-10-8-14(13)19-15(20-16)11-12-5-3-2-4-6-12/h2-6,18H,7-11H2,1H3,(H,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019662
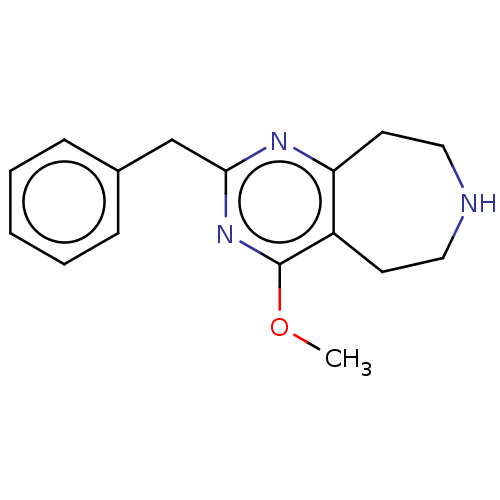
(CHEMBL3286560)Show InChI InChI=1S/C16H19N3O/c1-20-16-13-7-9-17-10-8-14(13)18-15(19-16)11-12-5-3-2-4-6-12/h2-6,17H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019691

(CHEMBL3286565)Show InChI InChI=1S/C17H22N4/c1-12(13-6-4-3-5-7-13)16-20-15-9-11-19-10-8-14(15)17(18-2)21-16/h3-7,12,19H,8-11H2,1-2H3,(H,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346870
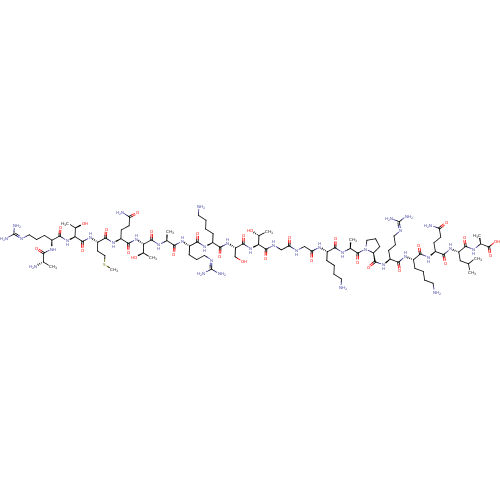
(CHEMBL1797647)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C93H169N35O28S/c1-45(2)41-62(83(148)113-49(6)90(155)156)123-79(144)59(28-30-65(98)133)119-75(140)54(22-12-15-34-95)117-77(142)57(25-18-37-107-92(102)103)121-85(150)64-27-20-39-128(64)89(154)48(5)112-74(139)53(21-11-14-33-94)114-68(136)43-109-67(135)42-110-86(151)69(50(7)130)125-84(149)63(44-129)124-78(143)55(23-13-16-35-96)118-76(141)56(24-17-36-106-91(100)101)116-73(138)47(4)111-87(152)70(51(8)131)126-82(147)60(29-31-66(99)134)120-80(145)61(32-40-157-10)122-88(153)71(52(9)132)127-81(146)58(115-72(137)46(3)97)26-19-38-108-93(104)105/h45-64,69-71,129-132H,11-44,94-97H2,1-10H3,(H2,98,133)(H2,99,134)(H,109,135)(H,110,151)(H,111,152)(H,112,139)(H,113,148)(H,114,136)(H,115,137)(H,116,138)(H,117,142)(H,118,141)(H,119,140)(H,120,145)(H,121,150)(H,122,153)(H,123,144)(H,124,143)(H,125,149)(H,126,147)(H,127,146)(H,155,156)(H4,100,101,106)(H4,102,103,107)(H4,104,105,108)/t46-,47-,48-,49-,50+,51+,52+,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,69-,70-,71-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A expressed in Escherichia coli using H3K4me as substrate by peroxidase coupled enzyme assay |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019692
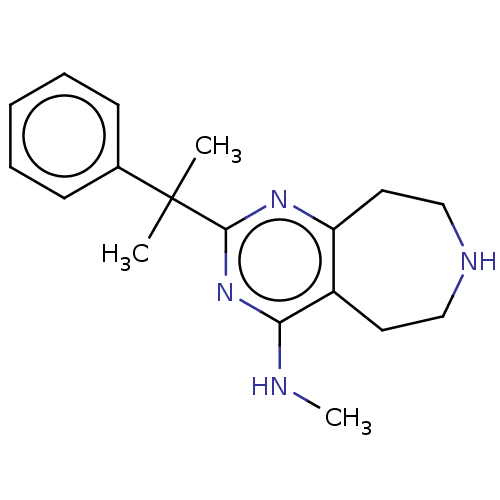
(CHEMBL3286566)Show InChI InChI=1S/C18H24N4/c1-18(2,13-7-5-4-6-8-13)17-21-15-10-12-20-11-9-14(15)16(19-3)22-17/h4-8,20H,9-12H2,1-3H3,(H,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM113742
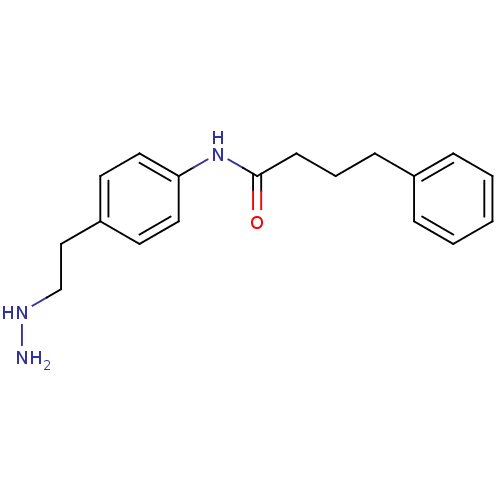
(N-[4-(2-Hydrazinylethyl)phenyl]-4-phenylbutanamide...)Show InChI InChI=1S/C18H23N3O/c19-20-14-13-16-9-11-17(12-10-16)21-18(22)8-4-7-15-5-2-1-3-6-15/h1-3,5-6,9-12,20H,4,7-8,13-14,19H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged KDM1A (unknown origin) expressed in Escherichia coli BL21-CodonPlus-(DE3)-RIPL cells using dimethyl-Lys-4 H3-21 as substrate... |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019695
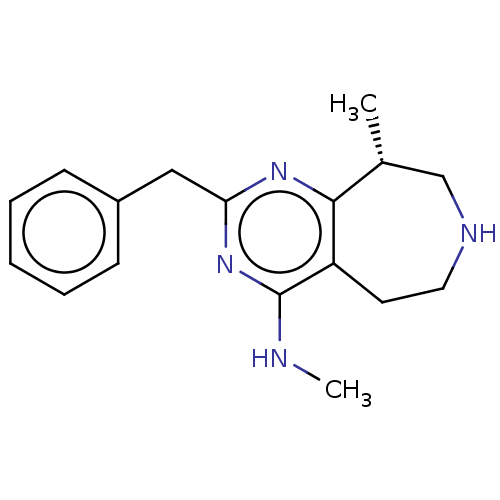
(CHEMBL3286555)Show InChI InChI=1S/C17H22N4/c1-12-11-19-9-8-14-16(12)20-15(21-17(14)18-2)10-13-6-4-3-5-7-13/h3-7,12,19H,8-11H2,1-2H3,(H,18,20,21)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50342539
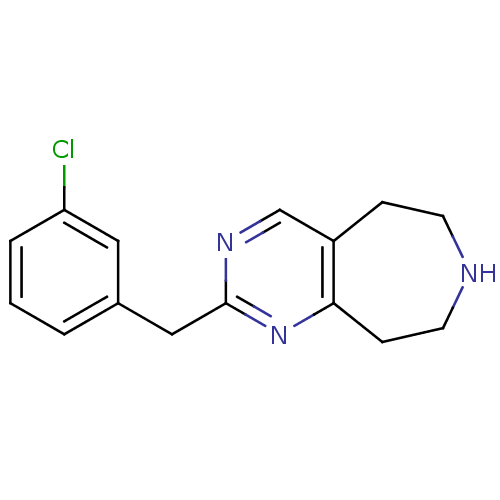
(2-(3-chlorobenzyl)-6,7,8,9-tetrahydro-5H-pyrimido[...)Show InChI InChI=1S/C15H16ClN3/c16-13-3-1-2-11(8-13)9-15-18-10-12-4-6-17-7-5-14(12)19-15/h1-3,8,10,17H,4-7,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human 5HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2715-20 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.120
BindingDB Entry DOI: 10.7270/Q2HD7VZZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019694
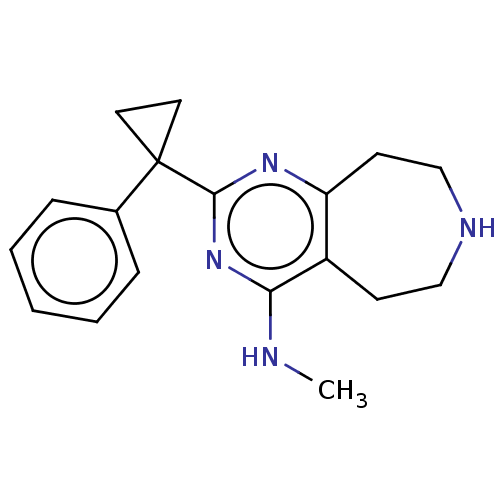
(CHEMBL3286554)Show InChI InChI=1S/C18H22N4/c1-19-16-14-7-11-20-12-8-15(14)21-17(22-16)18(9-10-18)13-5-3-2-4-6-13/h2-6,20H,7-12H2,1H3,(H,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158884

(CHEMBL3785550)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C51H87N17O10/c1-29(2)26-36(46(75)67-40(30(3)4)48(77)62-34(19-12-24-60-51(56)57)43(72)63-35(16-8-9-21-52)49(78)68-25-13-20-39(68)41(53)70)64-45(74)37(27-31-14-6-5-7-15-31)65-47(76)38(28-69)66-44(73)33(18-11-23-59-50(54)55)61-42(71)32-17-10-22-58-32/h5-7,14-15,29-30,32-40,58,69H,8-13,16-28,52H2,1-4H3,(H2,53,70)(H,61,71)(H,62,77)(H,63,72)(H,64,74)(H,65,76)(H,66,73)(H,67,75)(H4,54,55,59)(H4,56,57,60)/t32-,33-,34-,35-,36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged KDM1A (171 to 836 residues) using H3K4me2 as substrate by peroxidase coupled enzyme assay |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50342538
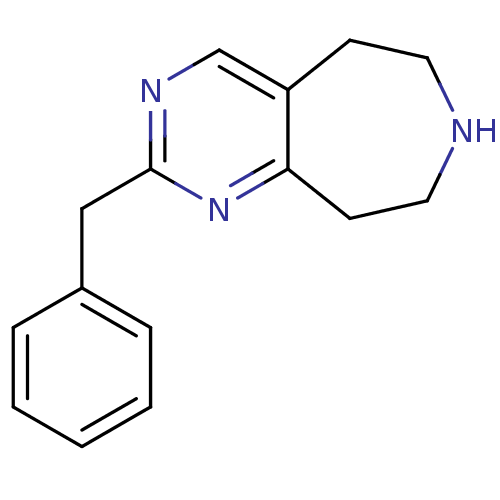
(2-benzyl-6,7,8,9-tetrahydro-5H-pyrimido[5,4-d]azep...)Show InChI InChI=1S/C15H17N3/c1-2-4-12(5-3-1)10-15-17-11-13-6-8-16-9-7-14(13)18-15/h1-5,11,16H,6-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50342538

(2-benzyl-6,7,8,9-tetrahydro-5H-pyrimido[5,4-d]azep...)Show InChI InChI=1S/C15H17N3/c1-2-4-12(5-3-1)10-15-17-11-13-6-8-16-9-7-14(13)18-15/h1-5,11,16H,6-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human 5HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2715-20 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.120
BindingDB Entry DOI: 10.7270/Q2HD7VZZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50161646

((1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-ben...)Show InChI InChI=1S/C11H14ClN/c1-8-7-13-5-4-9-2-3-10(12)6-11(8)9/h2-3,6,8,13H,4-5,7H2,1H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human 5HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2715-20 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.120
BindingDB Entry DOI: 10.7270/Q2HD7VZZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158882
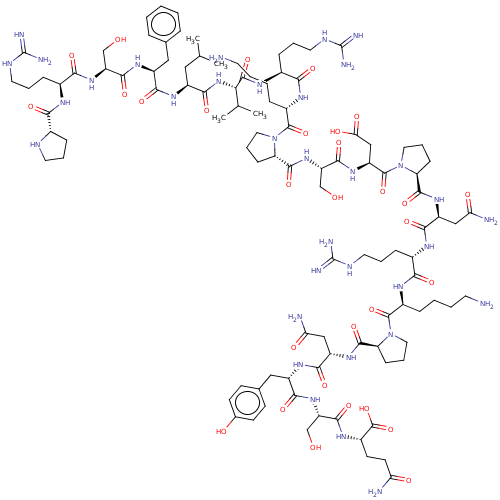
(CHEMBL3786209)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C105H168N34O30/c1-54(2)45-66(127-87(153)67(46-56-19-6-5-7-20-56)129-93(159)73(52-141)133-86(152)61(25-14-40-119-104(113)114)121-83(149)59-23-12-38-117-59)91(157)136-82(55(3)4)98(164)123-62(26-15-41-120-105(115)116)85(151)125-64(22-9-11-37-107)100(166)138-43-17-29-77(138)97(163)135-74(53-142)94(160)132-71(50-81(147)148)101(167)139-44-18-28-76(139)96(162)130-69(48-79(109)145)89(155)122-60(24-13-39-118-103(111)112)84(150)124-63(21-8-10-36-106)99(165)137-42-16-27-75(137)95(161)131-70(49-80(110)146)90(156)128-68(47-57-30-32-58(143)33-31-57)88(154)134-72(51-140)92(158)126-65(102(168)169)34-35-78(108)144/h5-7,19-20,30-33,54-55,59-77,82,117,140-143H,8-18,21-29,34-53,106-107H2,1-4H3,(H2,108,144)(H2,109,145)(H2,110,146)(H,121,149)(H,122,155)(H,123,164)(H,124,150)(H,125,151)(H,126,158)(H,127,153)(H,128,156)(H,129,159)(H,130,162)(H,131,161)(H,132,160)(H,133,152)(H,134,154)(H,135,163)(H,136,157)(H,147,148)(H,168,169)(H4,111,112,118)(H4,113,114,119)(H4,115,116,120)/t59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,82-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged KDM1A (171 to 836 residues) using H3K4me2 as substrate by peroxidase coupled enzyme assay |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019674
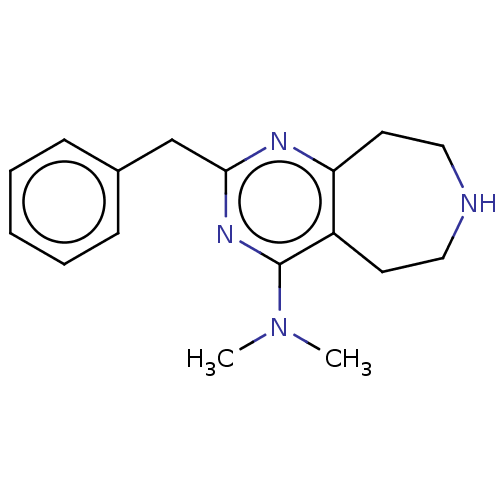
(CHEMBL3286563)Show InChI InChI=1S/C17H22N4/c1-21(2)17-14-8-10-18-11-9-15(14)19-16(20-17)12-13-6-4-3-5-7-13/h3-7,18H,8-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 233 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158883
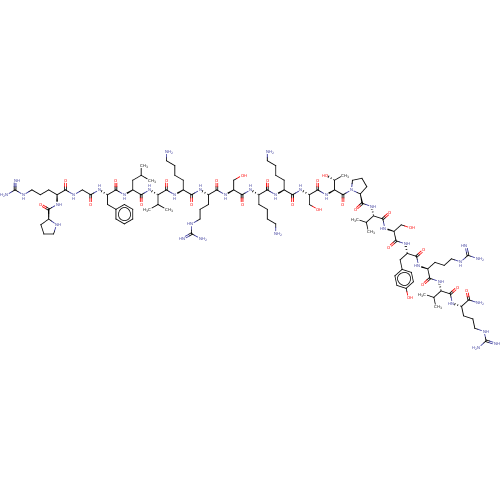
(CHEMBL3785964)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C106H182N36O25/c1-56(2)49-72(133-92(157)73(50-61-25-11-10-12-26-61)125-79(148)52-124-85(150)66(32-21-45-121-104(113)114)127-86(151)65-31-20-43-119-65)94(159)139-81(58(5)6)100(165)132-69(29-15-18-42-109)88(153)129-70(33-22-46-122-105(115)116)90(155)135-75(53-143)95(160)131-67(27-13-16-40-107)87(152)128-68(28-14-17-41-108)89(154)136-77(55-145)97(162)141-83(60(9)146)102(167)142-48-24-35-78(142)98(163)140-82(59(7)8)101(166)137-76(54-144)96(161)134-74(51-62-36-38-63(147)39-37-62)93(158)130-71(34-23-47-123-106(117)118)91(156)138-80(57(3)4)99(164)126-64(84(110)149)30-19-44-120-103(111)112/h10-12,25-26,36-39,56-60,64-78,80-83,119,143-147H,13-24,27-35,40-55,107-109H2,1-9H3,(H2,110,149)(H,124,150)(H,125,148)(H,126,164)(H,127,151)(H,128,152)(H,129,153)(H,130,158)(H,131,160)(H,132,165)(H,133,157)(H,134,161)(H,135,155)(H,136,154)(H,137,166)(H,138,156)(H,139,159)(H,140,163)(H,141,162)(H4,111,112,120)(H4,113,114,121)(H4,115,116,122)(H4,117,118,123)/t60-,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,75+,76+,77+,78+,80+,81+,82+,83+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged KDM1A (171 to 836 residues) using H3K4me2 as substrate by peroxidase coupled enzyme assay |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50342547
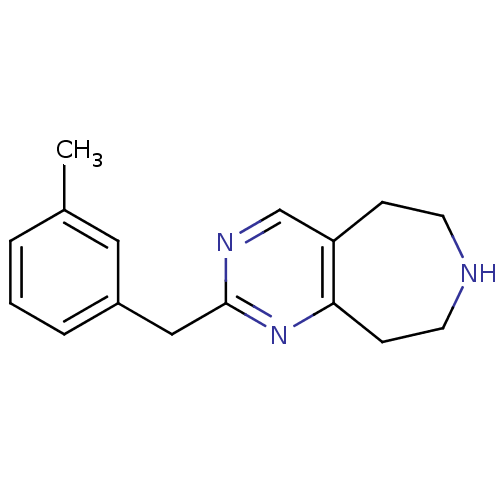
(2-(3-methylbenzyl)-6,7,8,9-tetrahydro-5H-pyrimido[...)Show InChI InChI=1S/C16H19N3/c1-12-3-2-4-13(9-12)10-16-18-11-14-5-7-17-8-6-15(14)19-16/h2-4,9,11,17H,5-8,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human 5HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2715-20 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.120
BindingDB Entry DOI: 10.7270/Q2HD7VZZ |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 2A
(Homo sapiens (Human)) | BDBM50158852

(CHEMBL3785340)Show SMILES [I-].CCCCCC[n+]1ccc(\C=C\c2ccc(cc2)N(C)C)cc1 Show InChI InChI=1S/C21H29N2.HI/c1-4-5-6-7-16-23-17-14-20(15-18-23)9-8-19-10-12-21(13-11-19)22(2)3;/h8-15,17-18H,4-7,16H2,1-3H3;1H/q+1;/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant His6-tagged KDM2A (1 to 517 residues) (unknown origin) expressed in Escherichia coli BL21 cells using methylsta... |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50342548
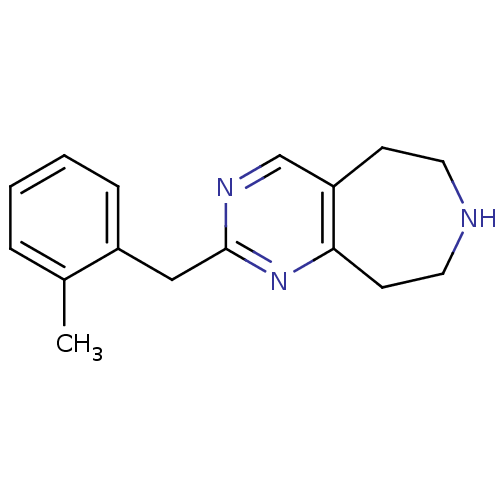
(2-(2-methylbenzyl)-6,7,8,9-tetrahydro-5H-pyrimido[...)Show InChI InChI=1S/C16H19N3/c1-12-4-2-3-5-13(12)10-16-18-11-14-6-8-17-9-7-15(14)19-16/h2-5,11,17H,6-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human 5HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2715-20 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.120
BindingDB Entry DOI: 10.7270/Q2HD7VZZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50342544

(2-(3-(trifluoromethyl)benzyl)-6,7,8,9-tetrahydro-5...)Show InChI InChI=1S/C16H16F3N3/c17-16(18,19)13-3-1-2-11(8-13)9-15-21-10-12-4-6-20-7-5-14(12)22-15/h1-3,8,10,20H,4-7,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human 5HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2715-20 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.120
BindingDB Entry DOI: 10.7270/Q2HD7VZZ |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 2A
(Homo sapiens (Human)) | BDBM50158851
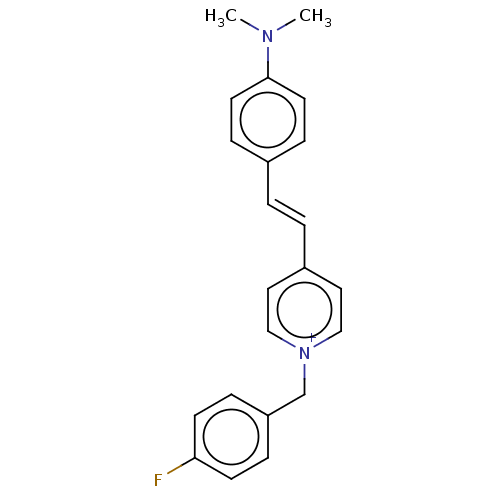
(CHEMBL3786265)Show SMILES [I-].CN(C)c1ccc(\C=C\c2cc[n+](Cc3ccc(F)cc3)cc2)cc1 Show InChI InChI=1S/C22H22FN2.HI/c1-24(2)22-11-7-18(8-12-22)3-4-19-13-15-25(16-14-19)17-20-5-9-21(23)10-6-20;/h3-16H,17H2,1-2H3;1H/q+1;/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant His6-tagged KDM2A (1 to 517 residues) (unknown origin) expressed in Escherichia coli BL21 cells using methylsta... |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50158852

(CHEMBL3785340)Show SMILES [I-].CCCCCC[n+]1ccc(\C=C\c2ccc(cc2)N(C)C)cc1 Show InChI InChI=1S/C21H29N2.HI/c1-4-5-6-7-16-23-17-14-20(15-18-23)9-8-19-10-12-21(13-11-19)22(2)3;/h8-15,17-18H,4-7,16H2,1-3H3;1H/q+1;/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant His6-tagged KDM4A (1 to 359 residues) (unknown origin) expressed in Escherichia coli BL21 cells using methylsta... |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50342549
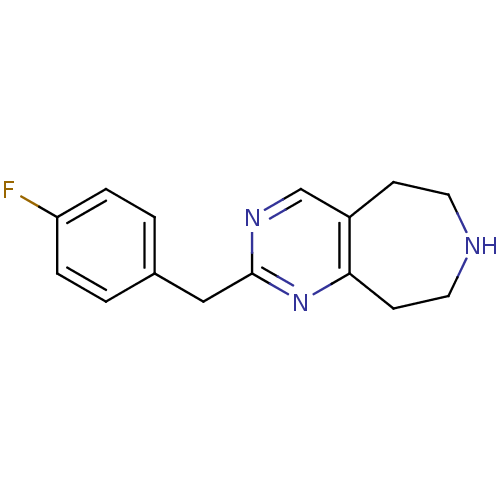
(2-(4-fluorobenzyl)-6,7,8,9-tetrahydro-5H-pyrimido[...)Show InChI InChI=1S/C15H16FN3/c16-13-3-1-11(2-4-13)9-15-18-10-12-5-7-17-8-6-14(12)19-15/h1-4,10,17H,5-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human 5HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2715-20 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.120
BindingDB Entry DOI: 10.7270/Q2HD7VZZ |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50158851

(CHEMBL3786265)Show SMILES [I-].CN(C)c1ccc(\C=C\c2cc[n+](Cc3ccc(F)cc3)cc2)cc1 Show InChI InChI=1S/C22H22FN2.HI/c1-24(2)22-11-7-18(8-12-22)3-4-19-13-15-25(16-14-19)17-20-5-9-21(23)10-6-20;/h3-16H,17H2,1-2H3;1H/q+1;/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant His6-tagged KDM4A (1 to 359 residues) (unknown origin) expressed in Escherichia coli BL21 cells using methylsta... |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019661
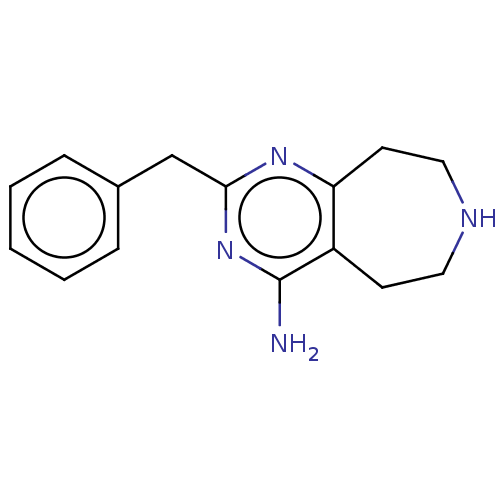
(CHEMBL3286559)Show InChI InChI=1S/C15H18N4/c16-15-12-6-8-17-9-7-13(12)18-14(19-15)10-11-4-2-1-3-5-11/h1-5,17H,6-10H2,(H2,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 958 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 2A
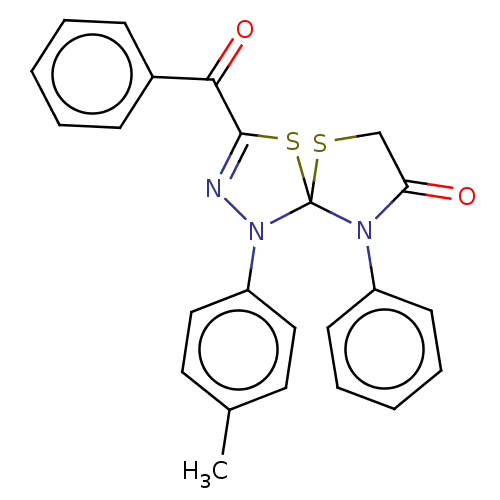
(Homo sapiens (Human)) | BDBM50158853
(CHEMBL3786188)Show SMILES Cc1ccc(cc1)N1N=C(SC11SCC(=O)N1c1ccccc1)C(=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H19N3O2S2/c1-17-12-14-20(15-13-17)27-24(26(21(28)16-30-24)19-10-6-3-7-11-19)31-23(25-27)22(29)18-8-4-2-5-9-18/h2-15H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant His6-tagged KDM2A (1 to 517 residues) (unknown origin) expressed in Escherichia coli BL21 cells using methylsta... |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158869

(CHEMBL3786182 | US10836743, Compound GSK-2879552 |...)Show SMILES OC(=O)c1ccc(CN2CCC(CN[C@@H]3C[C@H]3c3ccccc3)CC2)cc1 |r| Show InChI InChI=1S/C23H28N2O2/c26-23(27)20-8-6-18(7-9-20)16-25-12-10-17(11-13-25)15-24-22-14-21(22)19-4-2-1-3-5-19/h1-9,17,21-22,24H,10-16H2,(H,26,27)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of KDM1A (unknown origin) by peroxidase coupled assay |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158881
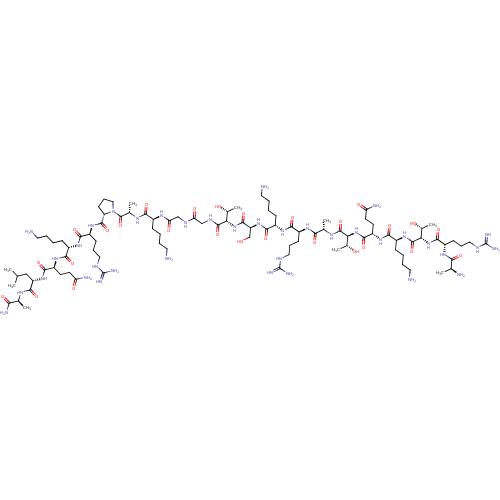
(CHEMBL3785549)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)CNC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)N)[C@@H](C)O)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C94H173N37O27/c1-46(2)42-63(85(152)114-48(4)73(102)140)126-82(149)61(30-32-66(100)136)122-77(144)55(23-11-15-35-96)120-80(147)59(27-19-39-110-93(105)106)124-87(154)65-29-21-41-131(65)91(158)50(6)116-76(143)54(22-10-14-34-95)117-69(139)44-112-68(138)43-113-88(155)70(51(7)133)128-86(153)64(45-132)127-81(148)56(24-12-16-36-97)121-79(146)58(26-18-38-109-92(103)104)119-75(142)49(5)115-89(156)71(52(8)134)129-84(151)62(31-33-67(101)137)123-78(145)57(25-13-17-37-98)125-90(157)72(53(9)135)130-83(150)60(118-74(141)47(3)99)28-20-40-111-94(107)108/h46-65,70-72,132-135H,10-45,95-99H2,1-9H3,(H2,100,136)(H2,101,137)(H2,102,140)(H,112,138)(H,113,155)(H,114,152)(H,115,156)(H,116,143)(H,117,139)(H,118,141)(H,119,142)(H,120,147)(H,121,146)(H,122,144)(H,123,145)(H,124,154)(H,125,157)(H,126,149)(H,127,148)(H,128,153)(H,129,151)(H,130,150)(H4,103,104,109)(H4,105,106,110)(H4,107,108,111)/t47-,48-,49-,50-,51+,52+,53+,54-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,70-,71-,72-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant KDM1A expressed in Escherichia coli using H3K4me as substrate by peroxidase coupled enzyme assay |
J Med Chem 59: 1308-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01758
BindingDB Entry DOI: 10.7270/Q2MP5559 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data