| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Chymotrypsinogen A |
|---|
| Ligand | BDBM50070010 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_49922 |
|---|
| IC50 | 1600±n/a nM |
|---|
| Citation |  Borthwick, AD; Weingarten, G; Haley, TM; Tomaszewski, M; Wang, W; Hu, Z; Bedard, J; Jin, H; Yuen, L; Mansour, TS Design and synthesis of monocyclic beta-lactams as mechanism-based inhibitors of human cytomegalovirus protease. Bioorg Med Chem Lett8:365-70 (1999) [PubMed] Borthwick, AD; Weingarten, G; Haley, TM; Tomaszewski, M; Wang, W; Hu, Z; Bedard, J; Jin, H; Yuen, L; Mansour, TS Design and synthesis of monocyclic beta-lactams as mechanism-based inhibitors of human cytomegalovirus protease. Bioorg Med Chem Lett8:365-70 (1999) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Chymotrypsinogen A |
|---|
| Name: | Chymotrypsinogen A |
|---|
| Synonyms: | Alpha-chymotrypsin | CTRA_BOVIN | Chymotrypsin A | Chymotrypsin A chain A | Chymotrypsin A chain B | Chymotrypsin A chain C | Chymotrypsinogen A | alpha-Chymotrypsin (α-Chymotrypsin) |
|---|
| Type: | Serine protease |
|---|
| Mol. Mass.: | 25670.88 |
|---|
| Organism: | Bos taurus (bovine) |
|---|
| Description: | n/a |
|---|
| Residue: | 245 |
|---|
| Sequence: | CGVPAIQPVLSGLSRIVNGEEAVPGSWPWQVSLQDKTGFHFCGGSLINENWVVTAAHCGV
TTSDVVVAGEFDQGSSSEKIQKLKIAKVFKNSKYNSLTINNDITLLKLSTAASFSQTVSA
VCLPSASDDFAAGTTCVTTGWGLTRYTNANTPDRLQQASLPLLSNTNCKKYWGTKIKDAM
ICAGASGVSSCMGDSGGPLVCKKNGAWTLVGIVSWGSSTCSTSTPGVYARVTALVNWVQQ
TLAAN
|
|
|
|---|
| BDBM50070010 |
|---|
| n/a |
|---|
| Name | BDBM50070010 |
|---|
| Synonyms: | 4-((2R,3R)-1-Benzylcarbamoyl-3-methyl-4-oxo-azetidin-2-yloxy)-benzoic acid | CHEMBL122575 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H18N2O5 |
|---|
| Mol. Mass. | 354.3566 |
|---|
| SMILES | C[C@@H]1[C@@H](Oc2ccc(cc2)C(O)=O)N(C(=O)NCc2ccccc2)C1=O |
|---|
| Structure | 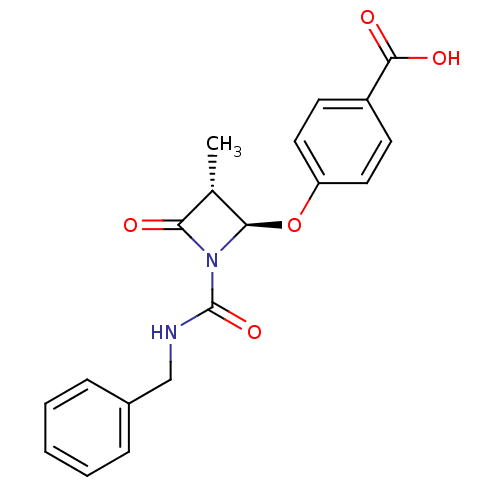
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Borthwick, AD; Weingarten, G; Haley, TM; Tomaszewski, M; Wang, W; Hu, Z; Bedard, J; Jin, H; Yuen, L; Mansour, TS Design and synthesis of monocyclic beta-lactams as mechanism-based inhibitors of human cytomegalovirus protease. Bioorg Med Chem Lett8:365-70 (1999) [PubMed]
Borthwick, AD; Weingarten, G; Haley, TM; Tomaszewski, M; Wang, W; Hu, Z; Bedard, J; Jin, H; Yuen, L; Mansour, TS Design and synthesis of monocyclic beta-lactams as mechanism-based inhibitors of human cytomegalovirus protease. Bioorg Med Chem Lett8:365-70 (1999) [PubMed]