

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50377655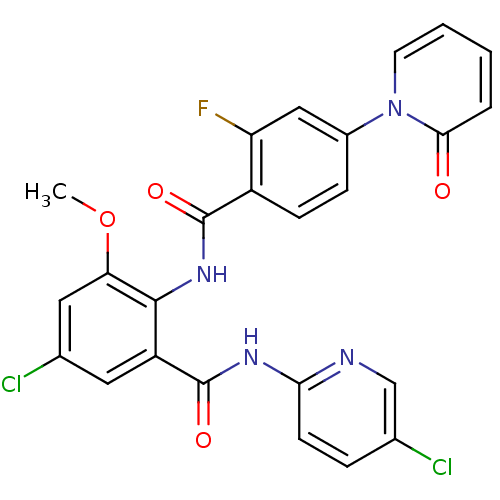 (CHEMBL260160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2845-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.092 BindingDB Entry DOI: 10.7270/Q2611169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50154364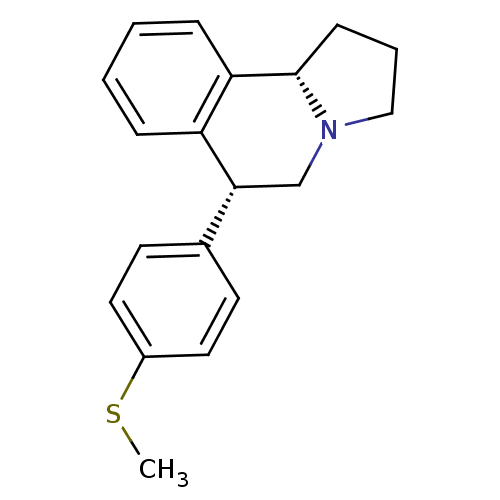 ((6R,10bS)-6-(4-Methylsulfanyl-phenyl)-1,2,3,5,6,10...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for serotonin transporter expresed in LCK PK1 cells | J Med Chem 47: 5258-64 (2004) Article DOI: 10.1021/jm049917p BindingDB Entry DOI: 10.7270/Q2QJ7GRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50110577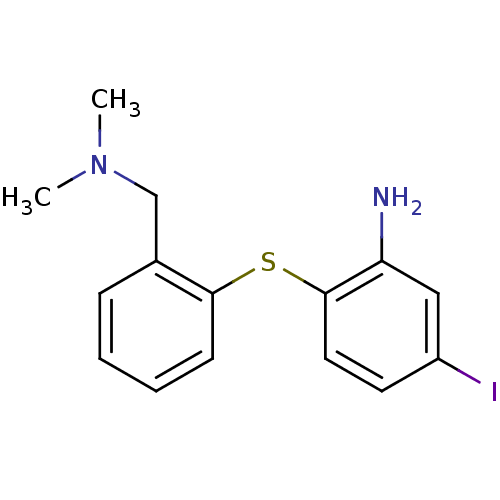 (2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity for serotonin transporter expresed in LCK PK1 cells | J Med Chem 47: 5258-64 (2004) Article DOI: 10.1021/jm049917p BindingDB Entry DOI: 10.7270/Q2QJ7GRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University Curated by ChEMBL | Assay Description Inhibition of Bcl2 (unknown origin) | Eur J Med Chem 177: 63-75 (2019) Article DOI: 10.1016/j.ejmech.2019.05.019 BindingDB Entry DOI: 10.7270/Q2R78JMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM484541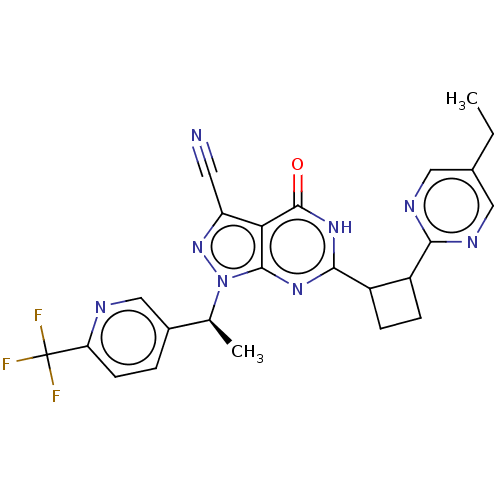 (US10934294, Example 62 | US11028092, Example 63) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... | US Patent US11028092 (2021) BindingDB Entry DOI: 10.7270/Q2XS5ZH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM484529 (US10934294, Example 50 | US10934294, Example 51 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... | US Patent US11028092 (2021) BindingDB Entry DOI: 10.7270/Q2XS5ZH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484529 (US10934294, Example 50 | US10934294, Example 51 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484541 (US10934294, Example 62 | US11028092, Example 63) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484570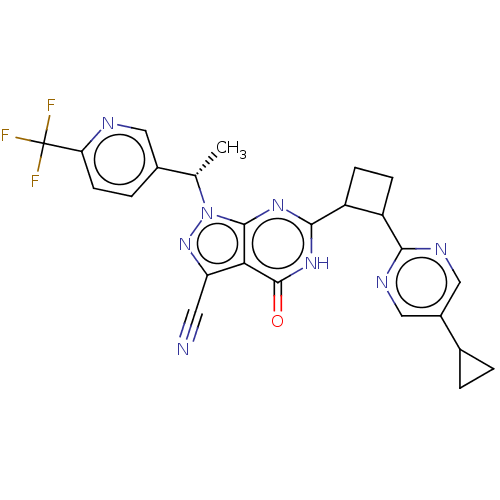 (US10934294, Example 91 | US10934294, Example 92 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50162797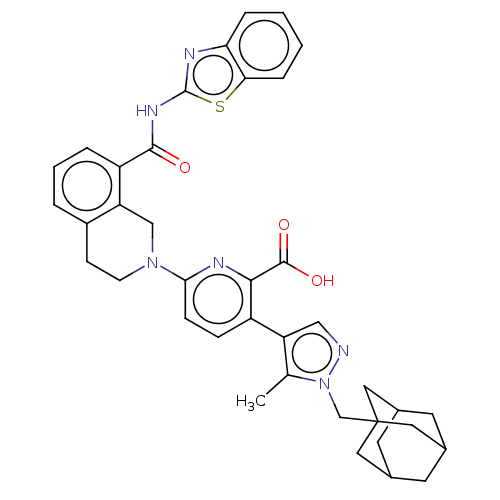 (CHEMBL3793424) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University Curated by ChEMBL | Assay Description Inhibition of Bcl-xL (unknown origin) | Eur J Med Chem 177: 63-75 (2019) Article DOI: 10.1016/j.ejmech.2019.05.019 BindingDB Entry DOI: 10.7270/Q2R78JMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484497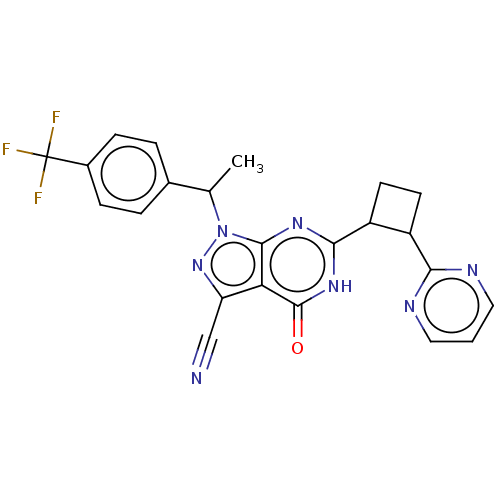 (US10934294, Example 19 | US10934294, Example 20 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM484570 (US10934294, Example 91 | US10934294, Example 92 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... | US Patent US11028092 (2021) BindingDB Entry DOI: 10.7270/Q2XS5ZH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM484497 (US10934294, Example 19 | US10934294, Example 20 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... | US Patent US11028092 (2021) BindingDB Entry DOI: 10.7270/Q2XS5ZH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50374877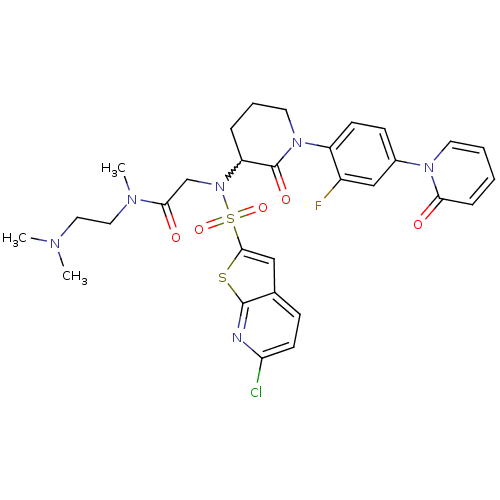 (CHEMBL270221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2428-33 (2008) Article DOI: 10.1016/j.bmcl.2008.02.054 BindingDB Entry DOI: 10.7270/Q2RX9CZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50377635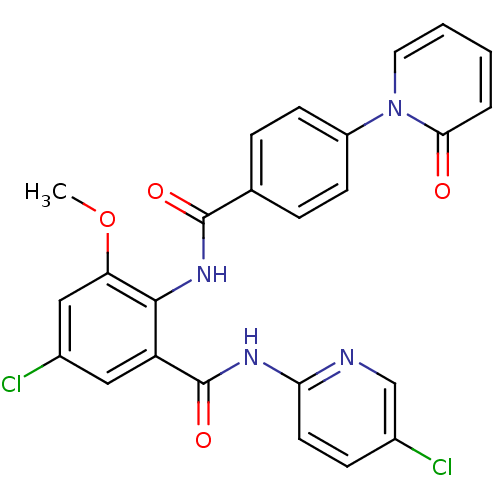 (CHEMBL402980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2845-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.092 BindingDB Entry DOI: 10.7270/Q2611169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50374879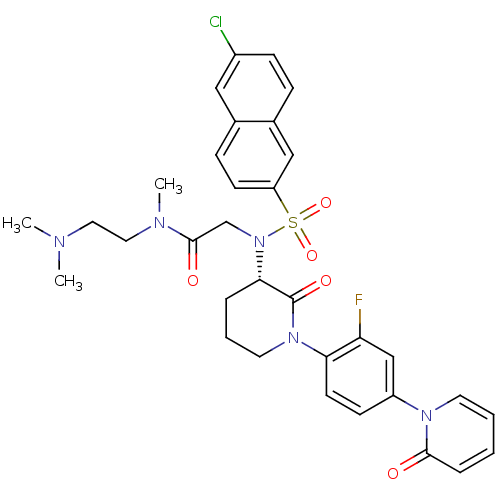 (CHEMBL401958) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2428-33 (2008) Article DOI: 10.1016/j.bmcl.2008.02.054 BindingDB Entry DOI: 10.7270/Q2RX9CZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM484551 (US10934294, Example 72 | US10934294, Example 73 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... | US Patent US11028092 (2021) BindingDB Entry DOI: 10.7270/Q2XS5ZH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50374878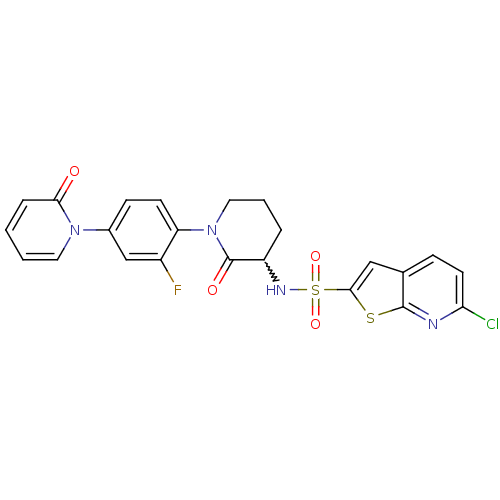 (CHEMBL270862) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2428-33 (2008) Article DOI: 10.1016/j.bmcl.2008.02.054 BindingDB Entry DOI: 10.7270/Q2RX9CZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50374879 (CHEMBL401958) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2428-33 (2008) Article DOI: 10.1016/j.bmcl.2008.02.054 BindingDB Entry DOI: 10.7270/Q2RX9CZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484551 (US10934294, Example 72 | US10934294, Example 73 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50377637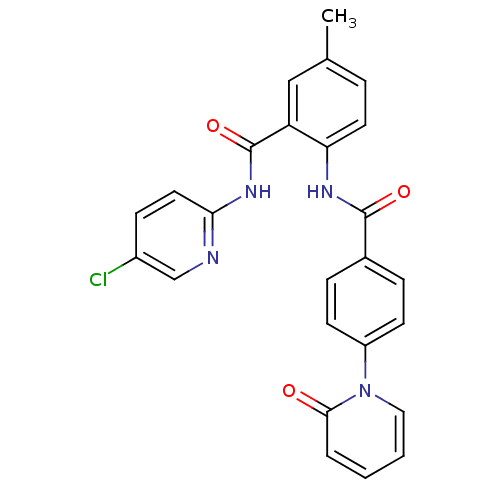 (CHEMBL257398) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2845-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.092 BindingDB Entry DOI: 10.7270/Q2611169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50475411 (CHEMBL197830) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM484573 (US10934294, Example 94 | US11028092, Example 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... | US Patent US11028092 (2021) BindingDB Entry DOI: 10.7270/Q2XS5ZH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484572 (US10934294, Example 93) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50475407 (CHEMBL198228) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50475410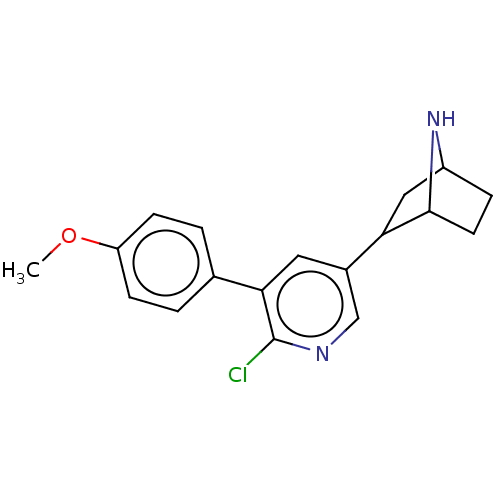 (CHEMBL372133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50475411 (CHEMBL197830) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha3-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50374876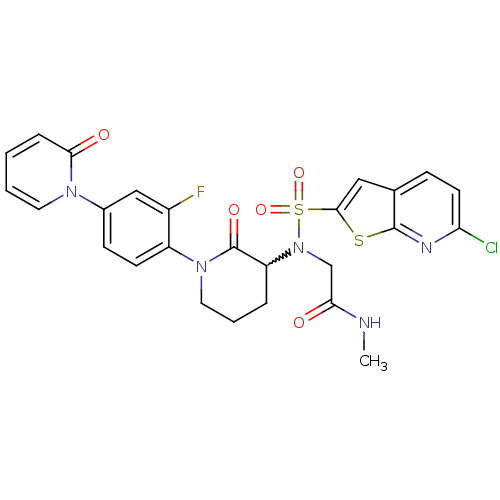 (CHEMBL270034) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2428-33 (2008) Article DOI: 10.1016/j.bmcl.2008.02.054 BindingDB Entry DOI: 10.7270/Q2RX9CZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50106490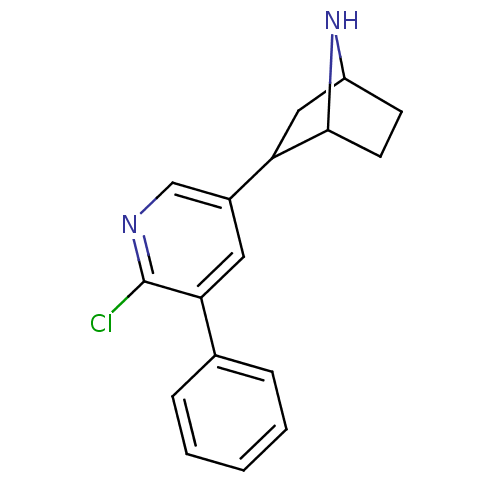 (2-(6-Chloro-5-phenyl-pyridin-3-yl)-7-aza-bicyclo[2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50171007 (4-[5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-2-fluoro-pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50106490 (2-(6-Chloro-5-phenyl-pyridin-3-yl)-7-aza-bicyclo[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha4-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50475410 (CHEMBL372133) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484596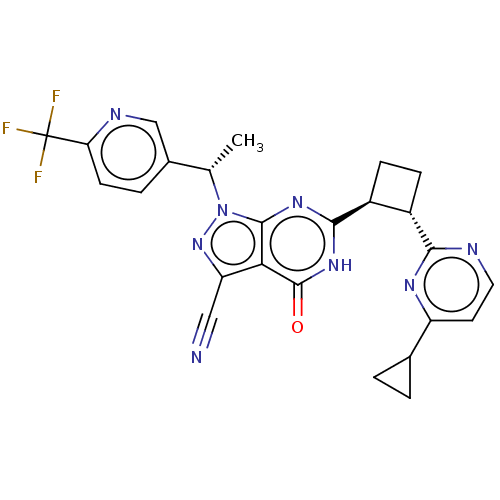 (US10934294, Example 114 | US11028092, Example 114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484593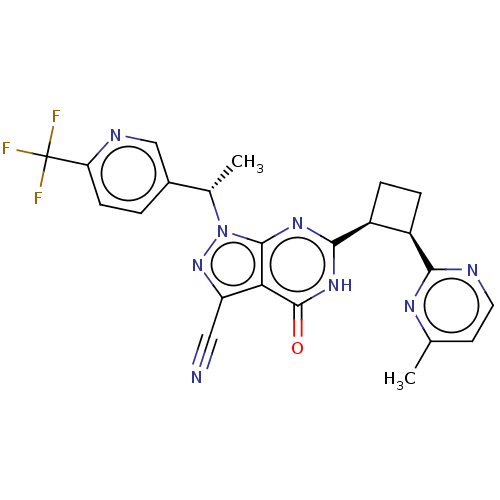 (US10934294, Example 111) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM484509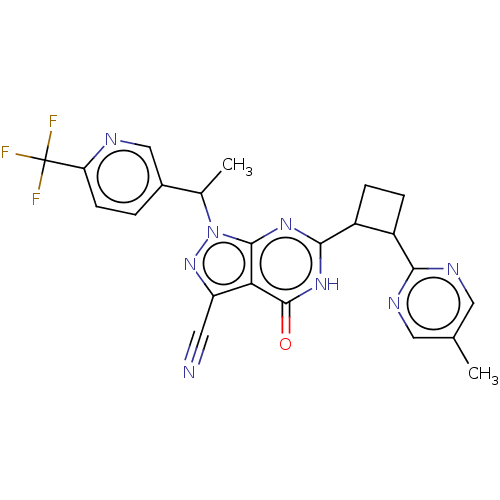 (US10934294, Example 31 | US10934294, Example 32 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... | US Patent US11028092 (2021) BindingDB Entry DOI: 10.7270/Q2XS5ZH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50096792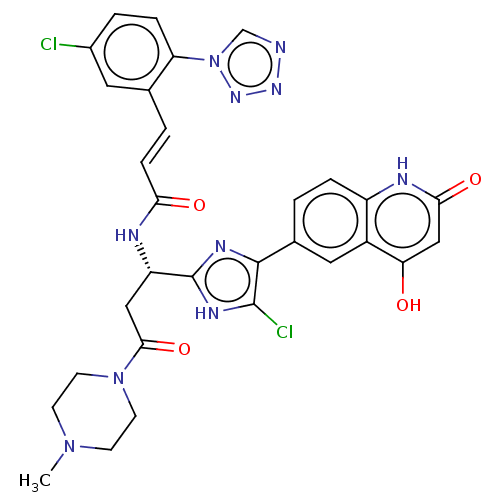 (CHEMBL3580759) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometry | ACS Med Chem Lett 6: 590-5 (2015) Article DOI: 10.1021/acsmedchemlett.5b00066 BindingDB Entry DOI: 10.7270/Q2B27X24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484509 (US10934294, Example 31 | US10934294, Example 32 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM502801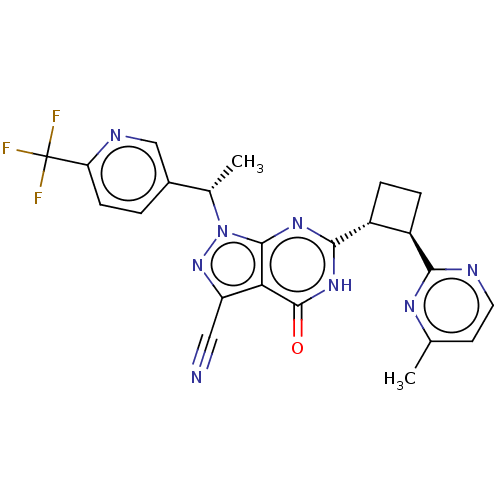 (US11028092, Example 111) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... | US Patent US11028092 (2021) BindingDB Entry DOI: 10.7270/Q2XS5ZH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM484596 (US10934294, Example 114 | US11028092, Example 114) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... | US Patent US11028092 (2021) BindingDB Entry DOI: 10.7270/Q2XS5ZH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50374871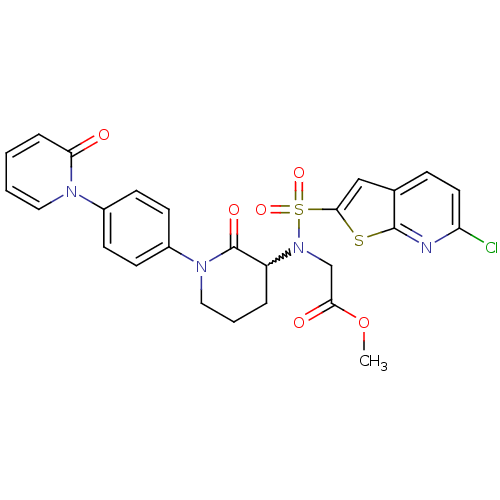 (CHEMBL258274) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2428-33 (2008) Article DOI: 10.1016/j.bmcl.2008.02.054 BindingDB Entry DOI: 10.7270/Q2RX9CZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50475409 (CHEMBL197526) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50062262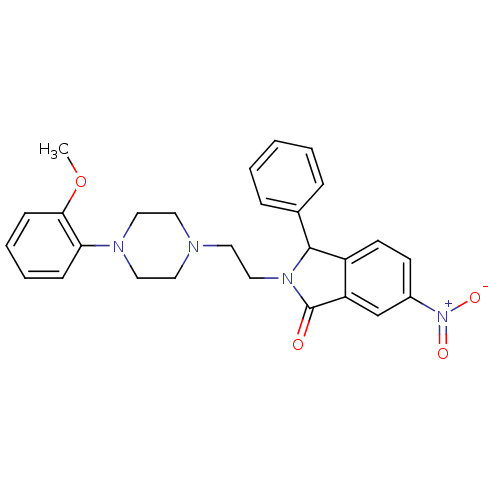 (2-{2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-ethyl}-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description In vitro binding affinity for 5-hydroxytryptamine 1A receptor in rat hippocampal membranes by [125I]-labeled agonist displacement. | J Med Chem 41: 157-66 (1998) Article DOI: 10.1021/jm970296s BindingDB Entry DOI: 10.7270/Q2TX3DH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50377629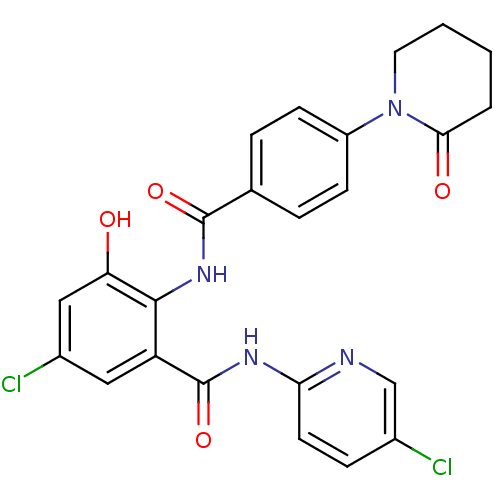 (CHEMBL260086) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2845-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.092 BindingDB Entry DOI: 10.7270/Q2611169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Homo sapiens (Human)) | BDBM484591 (US10934294, Example 110 | US11028092, Example 110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Assays for PDE 1 through 11 were performed in parallel at room temperature in 384-well microtiter plates with an incubation volume of 20.2 μL. S... | US Patent US11028092 (2021) BindingDB Entry DOI: 10.7270/Q2XS5ZH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50374875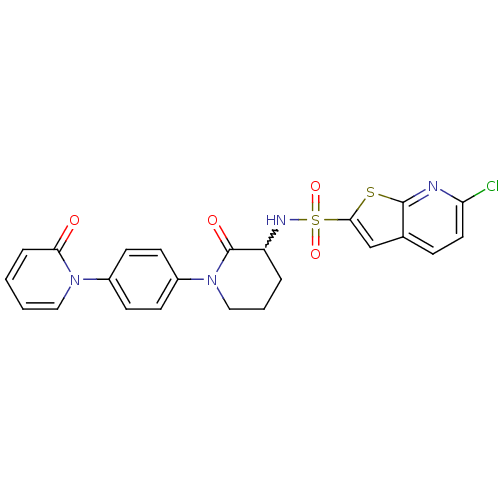 (CHEMBL269955) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2428-33 (2008) Article DOI: 10.1016/j.bmcl.2008.02.054 BindingDB Entry DOI: 10.7270/Q2RX9CZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484591 (US10934294, Example 110 | US11028092, Example 110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-2/beta-2 (Rattus norvegicus (Rat)) | BDBM50475407 (CHEMBL198228) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University College of Physicians and Surgeons Curated by ChEMBL | Assay Description In vitro binding affinity towards rat Nicotinic acetylcholine receptor alpha2-beta2 using 0.5 nM [3H]epibatidine | Bioorg Med Chem Lett 15: 4385-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.039 BindingDB Entry DOI: 10.7270/Q2H41V62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328717 (5-Chloro-N-(5-chloro-pyridin-2-yl)-3-methoxy-2-[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2845-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.092 BindingDB Entry DOI: 10.7270/Q2611169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 22270 total ) | Next | Last >> |