| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50073056 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_52555 (CHEMBL666254) |
|---|
| IC50 | 6000±n/a nM |
|---|
| Citation |  Bromidge, SM; Brown, AM; Clarke, SE; Dodgson, K; Gager, T; Grassam, HL; Jeffrey, PM; Joiner, GF; King, FD; Middlemiss, DN; Moss, SF; Newman, H; Riley, G; Routledge, C; Wyman, P 5-Chloro-N-(4-methoxy-3-piperazin-1-yl- phenyl)-3-methyl-2-benzothiophenesulfon- amide (SB-271046): a potent, selective, and orally bioavailable 5-HT6 receptor antagonist. J Med Chem42:202-5 (1999) [PubMed] Article Bromidge, SM; Brown, AM; Clarke, SE; Dodgson, K; Gager, T; Grassam, HL; Jeffrey, PM; Joiner, GF; King, FD; Middlemiss, DN; Moss, SF; Newman, H; Riley, G; Routledge, C; Wyman, P 5-Chloro-N-(4-methoxy-3-piperazin-1-yl- phenyl)-3-methyl-2-benzothiophenesulfon- amide (SB-271046): a potent, selective, and orally bioavailable 5-HT6 receptor antagonist. J Med Chem42:202-5 (1999) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50073056 |
|---|
| n/a |
|---|
| Name | BDBM50073056 |
|---|
| Synonyms: | 4-Bromo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulfonamide | CHEMBL292759 | SB-214111 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H22BrN3O3S |
|---|
| Mol. Mass. | 440.355 |
|---|
| SMILES | COc1ccc(NS(=O)(=O)c2ccc(Br)cc2)cc1N1CCN(C)CC1 |
|---|
| Structure | 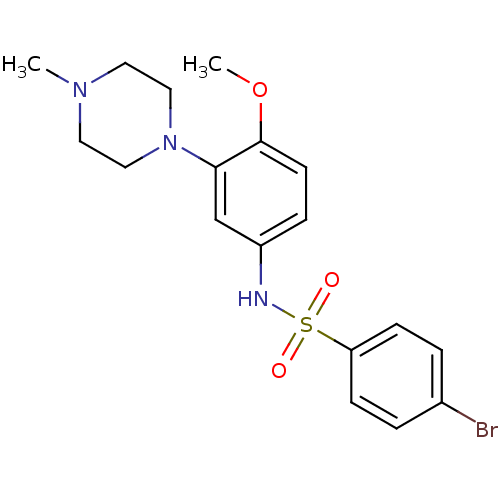
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bromidge, SM; Brown, AM; Clarke, SE; Dodgson, K; Gager, T; Grassam, HL; Jeffrey, PM; Joiner, GF; King, FD; Middlemiss, DN; Moss, SF; Newman, H; Riley, G; Routledge, C; Wyman, P 5-Chloro-N-(4-methoxy-3-piperazin-1-yl- phenyl)-3-methyl-2-benzothiophenesulfon- amide (SB-271046): a potent, selective, and orally bioavailable 5-HT6 receptor antagonist. J Med Chem42:202-5 (1999) [PubMed] Article
Bromidge, SM; Brown, AM; Clarke, SE; Dodgson, K; Gager, T; Grassam, HL; Jeffrey, PM; Joiner, GF; King, FD; Middlemiss, DN; Moss, SF; Newman, H; Riley, G; Routledge, C; Wyman, P 5-Chloro-N-(4-methoxy-3-piperazin-1-yl- phenyl)-3-methyl-2-benzothiophenesulfon- amide (SB-271046): a potent, selective, and orally bioavailable 5-HT6 receptor antagonist. J Med Chem42:202-5 (1999) [PubMed] Article