| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2A |
|---|
| Ligand | BDBM50010061 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1888740 (CHEMBL4390417) |
|---|
| Ki | 3.7±n/a nM |
|---|
| Citation |  Zar?ba, P; Ja?kowska, J; Czekaj, I; Sata?a, G Design, synthesis and molecular modelling of new bulky Fananserin derivatives with altered pharmacological profile as potential antidepressants. Bioorg Med Chem27:3396-3407 (2019) [PubMed] Article Zar?ba, P; Ja?kowska, J; Czekaj, I; Sata?a, G Design, synthesis and molecular modelling of new bulky Fananserin derivatives with altered pharmacological profile as potential antidepressants. Bioorg Med Chem27:3396-3407 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2A |
|---|
| Name: | 5-hydroxytryptamine receptor 2A |
|---|
| Synonyms: | 5-HT-2A | 5-HT2 | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_RAT | Htr2 | Htr2a | Serotonin Receptor 2A |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 52852.05 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Rat cortex membranes 5-HT2A receptors. |
|---|
| Residue: | 471 |
|---|
| Sequence: | MEILCEDNISLSSIPNSLMQLGDGPRLYHNDFNSRDANTSEASNWTIDAENRTNLSCEGY
LPPTCLSILHLQEKNWSALLTTVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIAD
MLLGFLVMPVSMLTILYGYRWPLPSKLCAIWIYLDVLFSTASIMHLCAISLDRYVAIQNP
IHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSF
VAFFIPLTIMVITYFLTIKSLQKEATLCVSDLSTRAKLASFSFLPQSSLSSEKLFQRSIH
REPGSYAGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNENVIGA
LLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENRKPLQLILVNTIPALAYK
SSQLQVGQKKNSQEDAEQTVDDCSMVTLGKQQSEENCTDNIETVNEKVSCV
|
|
|
|---|
| BDBM50010061 |
|---|
| n/a |
|---|
| Name | BDBM50010061 |
|---|
| Synonyms: | 2-{4-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-butyl}-2H-naphtho[1,8-cd]isothiazole 1,1-dioxide | CHEMBL82826 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H26FN3O2S |
|---|
| Mol. Mass. | 439.546 |
|---|
| SMILES | Fc1ccc(cc1)N1CCN(CCCCN2c3cccc4cccc(c34)S2(=O)=O)CC1 |
|---|
| Structure | 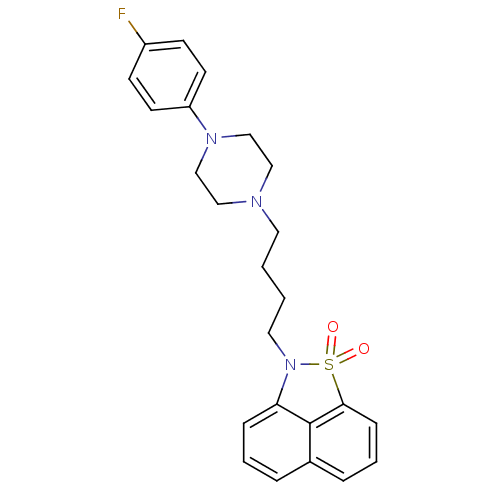
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zar?ba, P; Ja?kowska, J; Czekaj, I; Sata?a, G Design, synthesis and molecular modelling of new bulky Fananserin derivatives with altered pharmacological profile as potential antidepressants. Bioorg Med Chem27:3396-3407 (2019) [PubMed] Article
Zar?ba, P; Ja?kowska, J; Czekaj, I; Sata?a, G Design, synthesis and molecular modelling of new bulky Fananserin derivatives with altered pharmacological profile as potential antidepressants. Bioorg Med Chem27:3396-3407 (2019) [PubMed] Article