| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholinesterase |
|---|
| Ligand | BDBM50523572 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1890732 (CHEMBL4392486) |
|---|
| IC50 | 14220±n/a nM |
|---|
| Citation |  Arumugam, N; Almansour, AI; Kumar, RS; Kotresha, D; Saiswaroop, R; Venketesh, S Dispiropyrrolidinyl-piperidone embedded indeno[1,2-b]quinoxaline heterocyclic hybrids: Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg Med Chem27:2621-2628 (2019) [PubMed] Article Arumugam, N; Almansour, AI; Kumar, RS; Kotresha, D; Saiswaroop, R; Venketesh, S Dispiropyrrolidinyl-piperidone embedded indeno[1,2-b]quinoxaline heterocyclic hybrids: Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg Med Chem27:2621-2628 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholinesterase |
|---|
| Name: | Cholinesterase |
|---|
| Synonyms: | BCHE | Butyrylcholinesterase (BuChE) | CHLE_HORSE | Cholinesterase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 65643.35 |
|---|
| Organism: | Equus caballus (Horse) |
|---|
| Description: | P81908 |
|---|
| Residue: | 574 |
|---|
| Sequence: | EEDIIITTKNGKVRGMNLPVLGGTVTAFLGIPYAQPPLGRLRFKKPQSLTKWSNIWNATK
YANSCYQNTDQSFPGFLGSEMWNPNTELSEDCLYLNVWIPAPKPKNATVMIWIYGGGFQT
GTSSLPVYDGKFLARVERVIVVSMNYRVGALGFLALSENPEAPGNMGLFDQQLALQWVQK
NIAAFGGNPRSVTLFGESAGAASVSLHLLSPRSQPLFTRAILQSGSSNAPWAVTSLYEAR
NRTLTLAKRMGCSRDNETEMIKCLRDKDPQEILLNEVFVVPYDTLLSVNFGPTVDGDFLT
DMPDTLLQLGQFKRTQILVGVNKDEGTAFLVYGAPGFSKDNNSIITRKEFQEGLKIFFPR
VSEFGRESILFHYMDWLDDQRAENYREALDDVVGDYNIICPALEFTRKFSELGNDAFFYY
FEHRSTKLPWPEWMGVMHGYEIEFVFGLPLERRVNYTRAEEILSRSIMKRWANFAKYGNP
NGTQNNSTRWPVFKSTEQKYLTLNTESPKVYTKLRAQQCRFWTLFFPKVLELTGNIDEAE
REWKAGFHRWNNYMMDWKNQFNDYTSKKESCSDF
|
|
|
|---|
| BDBM50523572 |
|---|
| n/a |
|---|
| Name | BDBM50523572 |
|---|
| Synonyms: | CHEMBL4549715 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C44H37N5O |
|---|
| Mol. Mass. | 651.7975 |
|---|
| SMILES | O=C1\C(CNCC11C(C(Cc2c[nH]c3ccccc23)NC11C2N=c3ccccc3=NC2c2ccccc12)c1ccccc1)=C\c1ccccc1 |c:33,t:26| |
|---|
| Structure | 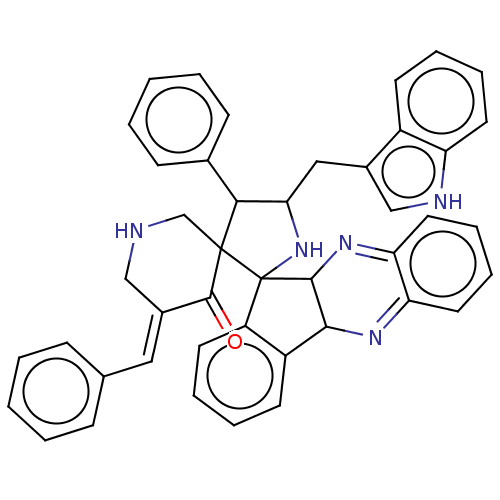
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Arumugam, N; Almansour, AI; Kumar, RS; Kotresha, D; Saiswaroop, R; Venketesh, S Dispiropyrrolidinyl-piperidone embedded indeno[1,2-b]quinoxaline heterocyclic hybrids: Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg Med Chem27:2621-2628 (2019) [PubMed] Article
Arumugam, N; Almansour, AI; Kumar, RS; Kotresha, D; Saiswaroop, R; Venketesh, S Dispiropyrrolidinyl-piperidone embedded indeno[1,2-b]quinoxaline heterocyclic hybrids: Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg Med Chem27:2621-2628 (2019) [PubMed] Article