

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50523570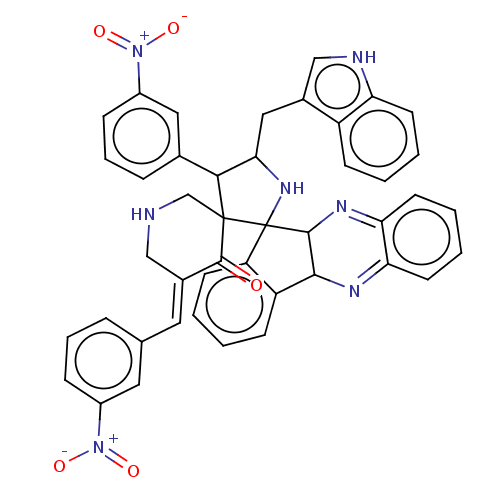 (CHEMBL4436182) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537713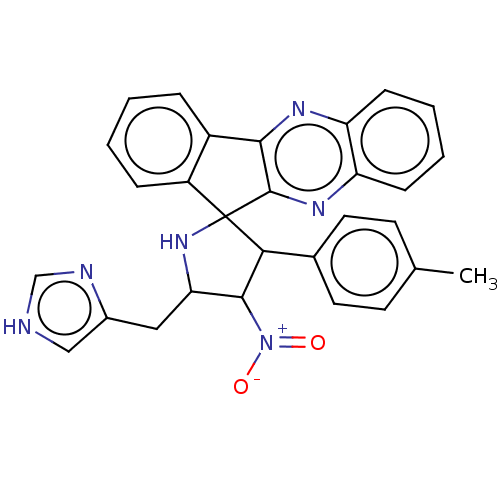 (CHEMBL4639749) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of human Protein kinase C gamma | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537716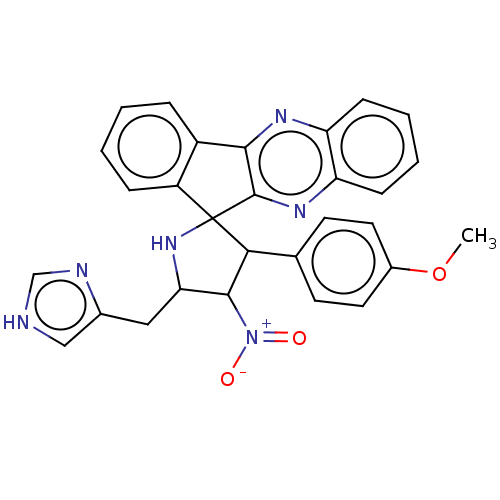 (CHEMBL4642244) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 2 | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C epsilon | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50523571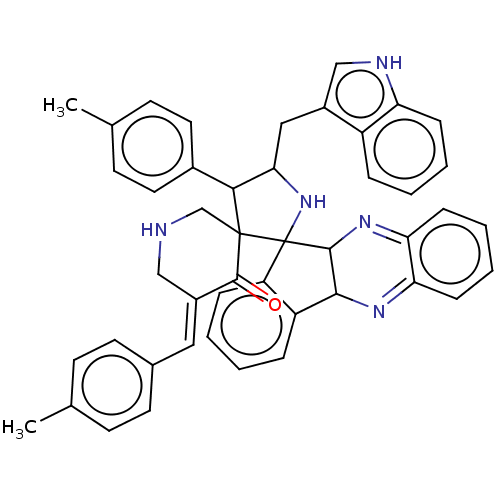 (CHEMBL4522515) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537714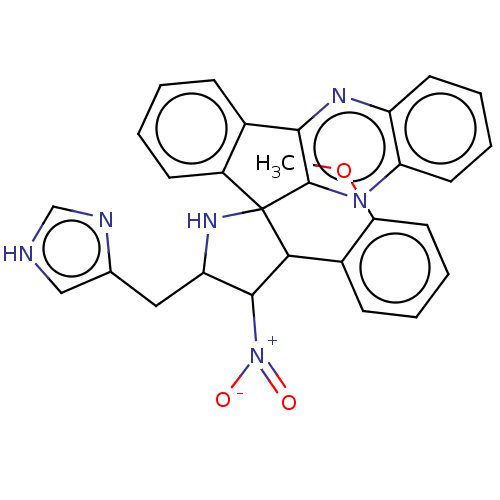 (CHEMBL4642959) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of human Protein kinase C beta 1 | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50523579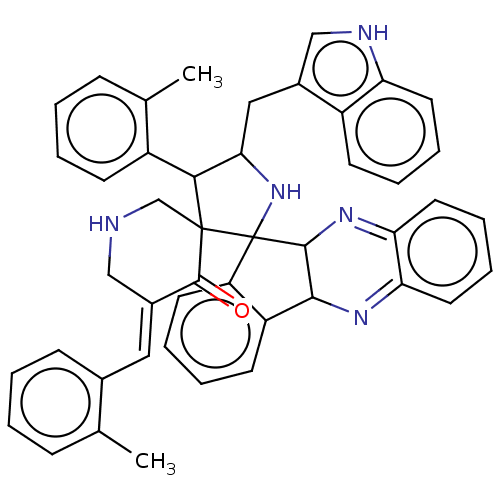 (CHEMBL4473000) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50523576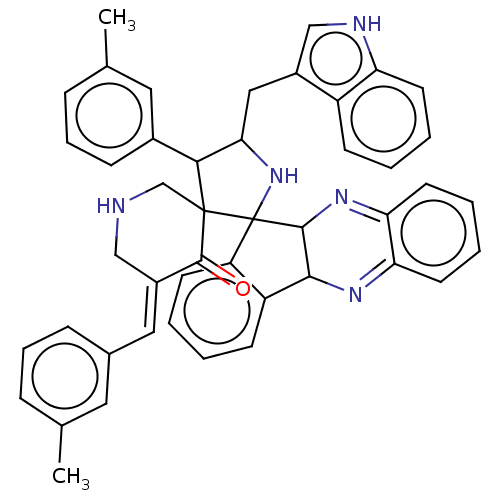 (CHEMBL4459290) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50523577 (CHEMBL4452994) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50523572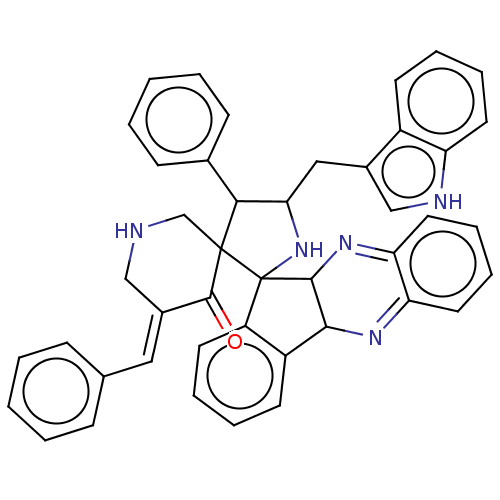 (CHEMBL4549715) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537712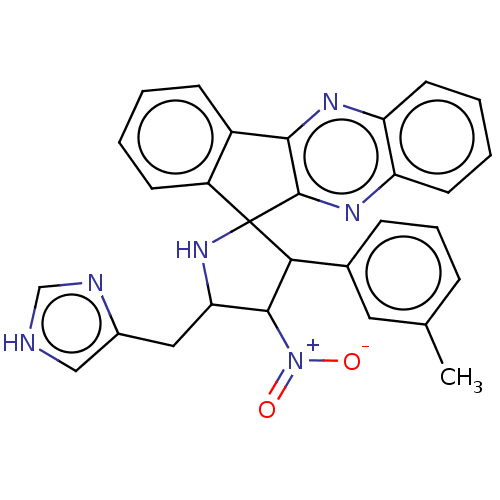 (CHEMBL4633851) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of human Protein kinase C beta 1 | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537715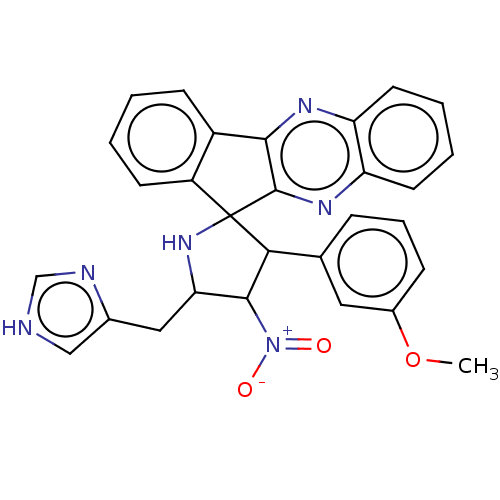 (CHEMBL4639357) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of human Protein kinase C beta 1 | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537711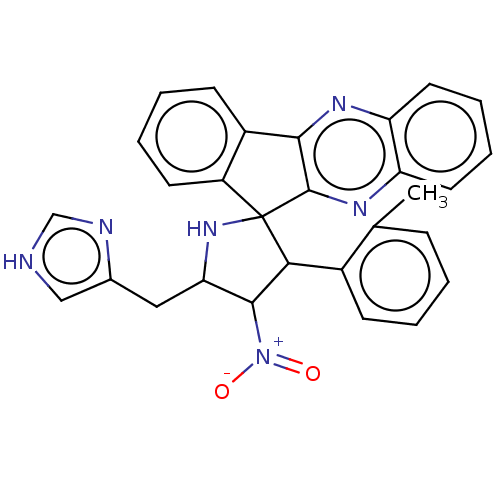 (CHEMBL4644961) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of human Protein kinase C gamma | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50523574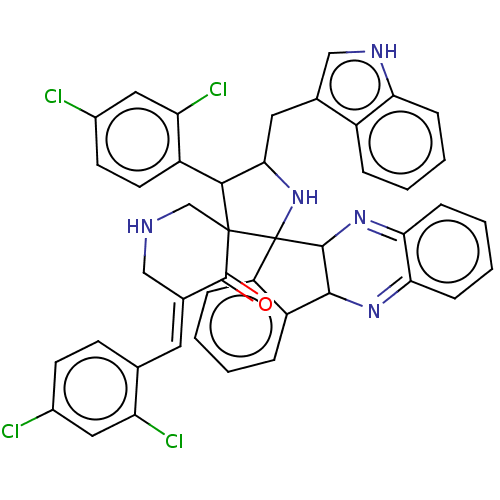 (CHEMBL4562781) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50523570 (CHEMBL4436182) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537716 (CHEMBL4642244) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C epsilon | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537708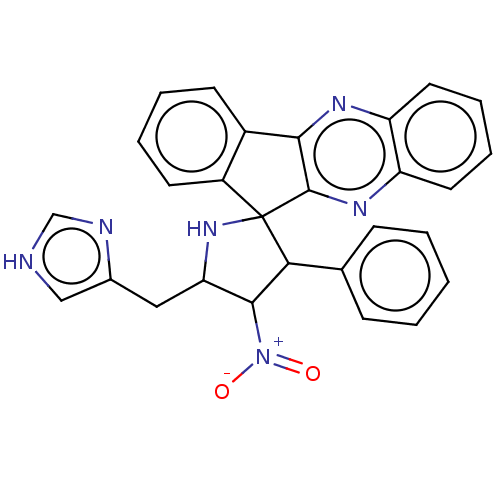 (CHEMBL4632770) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C zeta | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50523571 (CHEMBL4522515) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537713 (CHEMBL4639749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C delta | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50523575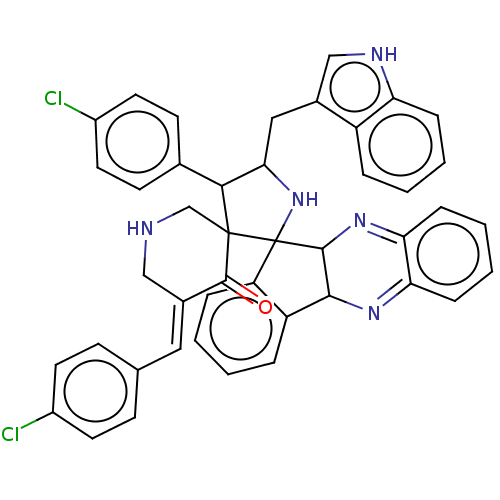 (CHEMBL4452219) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537712 (CHEMBL4633851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition Protein kinase C gamma | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537711 (CHEMBL4644961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C epsilon | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537714 (CHEMBL4642959) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 1 | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50523572 (CHEMBL4549715) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537717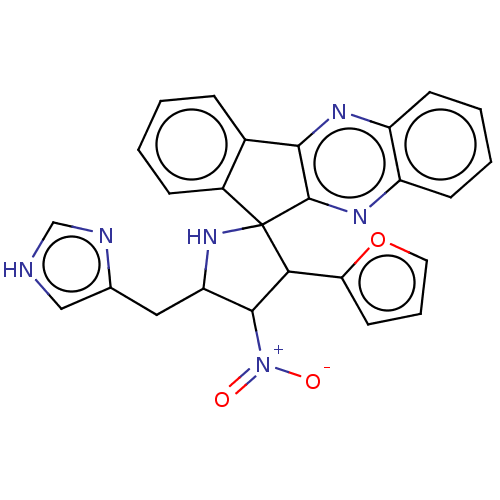 (CHEMBL4632994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition Protein kinase C gamma | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50523579 (CHEMBL4473000) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537715 (CHEMBL4639357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C delta | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50523576 (CHEMBL4459290) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50523578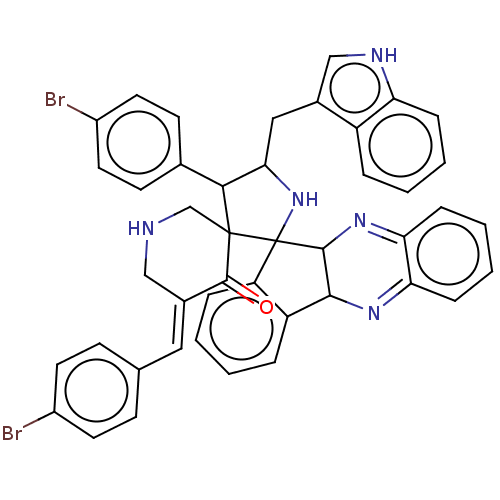 (CHEMBL4464716) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537708 (CHEMBL4632770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C epsilon | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537709 (CHEMBL4648224) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of human Protein kinase C epsilon | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50523573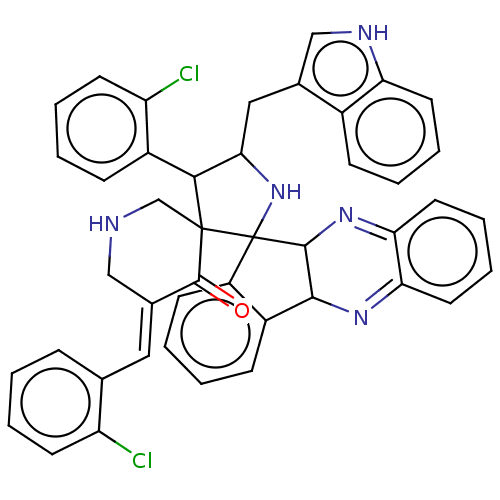 (CHEMBL4583817) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition and measured afte... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537718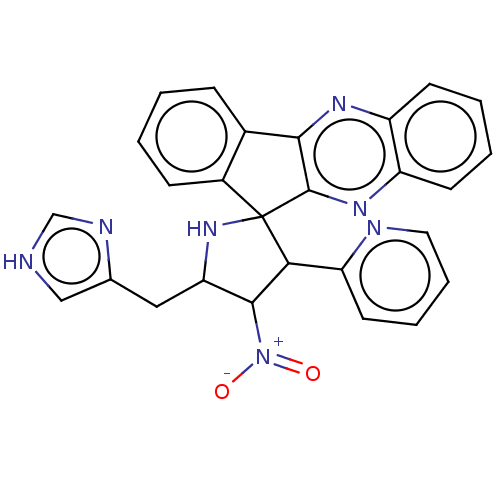 (CHEMBL4636399) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C delta | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50523574 (CHEMBL4562781) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50537710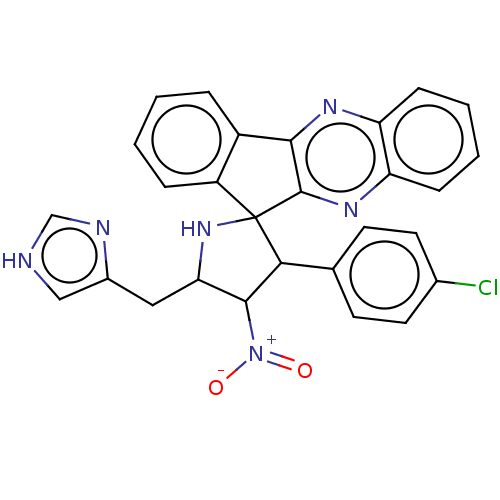 (CHEMBL4646153) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C zeta | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50523575 (CHEMBL4452219) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 1 | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50523578 (CHEMBL4464716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537710 (CHEMBL4646153) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 1 | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537717 (CHEMBL4632994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C zeta | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50523577 (CHEMBL4452994) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537718 (CHEMBL4636399) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C zeta | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50537709 (CHEMBL4648224) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition Protein kinase C gamma | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126789 BindingDB Entry DOI: 10.7270/Q2NC64QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50523573 (CHEMBL4583817) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured a... | Bioorg Med Chem 27: 2621-2628 (2019) Article DOI: 10.1016/j.bmc.2019.03.058 BindingDB Entry DOI: 10.7270/Q26W9FGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||