| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Lactoylglutathione lyase |
|---|
| Ligand | BDBM50092824 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1901790 (CHEMBL4404012) |
|---|
| Ki | 46±n/a nM |
|---|
| Citation |  Jin, T; Zhao, L; Wang, HP; Huang, ML; Yue, Y; Lu, C; Zheng, ZB Recent advances in the discovery and development of glyoxalase I inhibitors. Bioorg Med Chem28:0 (2020) [PubMed] Article Jin, T; Zhao, L; Wang, HP; Huang, ML; Yue, Y; Lu, C; Zheng, ZB Recent advances in the discovery and development of glyoxalase I inhibitors. Bioorg Med Chem28:0 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Lactoylglutathione lyase |
|---|
| Name: | Lactoylglutathione lyase |
|---|
| Synonyms: | Aldoketomutase | GLO1 | Glx I | Glyoxalase 1 (GLO1) | Glyoxalase I | Ketone-aldehyde mutase | LGUL_HUMAN | Methylglyoxalase | S-D-lactoylglutathione methylglyoxal lyase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 20772.95 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q04760 |
|---|
| Residue: | 184 |
|---|
| Sequence: | MAEPQPPSGGLTDEAALSCCSDADPSTKDFLLQQTMLRVKDPKKSLDFYTRVLGMTLIQK
CDFPIMKFSLYFLAYEDKNDIPKEKDEKIAWALSRKATLELTHNWGTEDDETQSYHNGNS
DPRGFGHIGIAVPDVYSACKRFEELGVKFVKKPDDGKMKGLAFIQDPDGYWIEILNPNKM
ATLM
|
|
|
|---|
| BDBM50092824 |
|---|
| n/a |
|---|
| Name | BDBM50092824 |
|---|
| Synonyms: | CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamoyl)glutathione |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H21ClN4O8S |
|---|
| Mol. Mass. | 476.889 |
|---|
| SMILES | N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O |
|---|
| Structure | 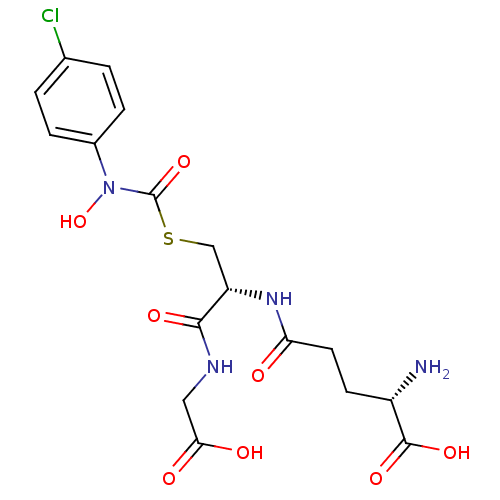
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jin, T; Zhao, L; Wang, HP; Huang, ML; Yue, Y; Lu, C; Zheng, ZB Recent advances in the discovery and development of glyoxalase I inhibitors. Bioorg Med Chem28:0 (2020) [PubMed] Article
Jin, T; Zhao, L; Wang, HP; Huang, ML; Yue, Y; Lu, C; Zheng, ZB Recent advances in the discovery and development of glyoxalase I inhibitors. Bioorg Med Chem28:0 (2020) [PubMed] Article