 Found 1745 hits with Last Name = 'zheng' and Initial = 'zb'
Found 1745 hits with Last Name = 'zheng' and Initial = 'zb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126960
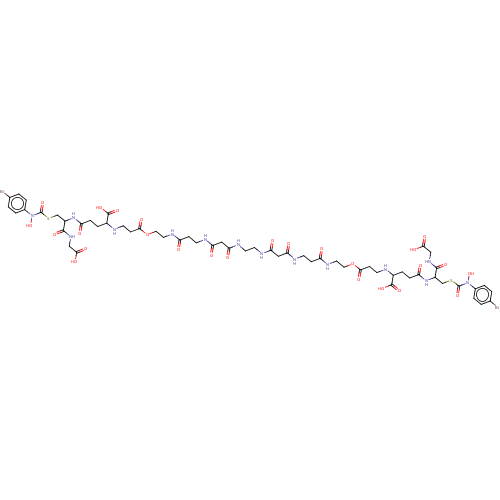
(CHEMBL3629116)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCNC(=O)CCNC(=O)CC(=O)NCCNC(=O)CC(=O)NCCC(=O)NCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C58H78Br2N14O26S2/c59-33-1-5-35(6-2-33)73(97)57(95)101-31-39(53(89)69-29-49(83)84)71-43(77)11-9-37(55(91)92)61-19-15-51(87)99-25-23-67-41(75)13-17-63-45(79)27-47(81)65-21-22-66-48(82)28-46(80)64-18-14-42(76)68-24-26-100-52(88)16-20-62-38(56(93)94)10-12-44(78)72-40(54(90)70-30-50(85)86)32-102-58(96)74(98)36-7-3-34(60)4-8-36/h1-8,37-40,61-62,97-98H,9-32H2,(H,63,79)(H,64,80)(H,65,81)(H,66,82)(H,67,75)(H,68,76)(H,69,89)(H,70,90)(H,71,77)(H,72,78)(H,83,84)(H,85,86)(H,91,92)(H,93,94) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ... |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526945
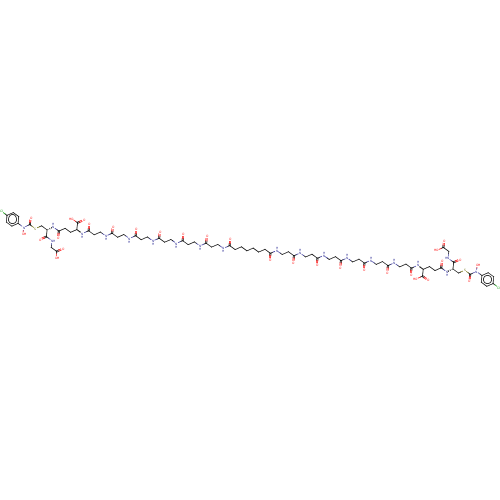
(CHEMBL4473806)Show SMILES ON(C(=O)SC[C@H](NC(=O)CC[C@H](NC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCNC(=O)CCCCCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)NCCC(=O)N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C78H112Cl2N20O30S2/c79-47-7-11-49(12-8-47)99(129)77(127)131-45-53(73(121)93-43-71(117)118)97-67(113)17-15-51(75(123)124)95-69(115)29-41-91-65(111)27-39-89-63(109)25-37-87-61(107)23-35-85-59(105)21-33-83-57(103)19-31-81-55(101)5-3-1-2-4-6-56(102)82-32-20-58(104)84-34-22-60(106)86-36-24-62(108)88-38-26-64(110)90-40-28-66(112)92-42-30-70(116)96-52(76(125)126)16-18-68(114)98-54(74(122)94-44-72(119)120)46-132-78(128)100(130)50-13-9-48(80)10-14-50/h7-14,51-54,129-130H,1-6,15-46H2,(H,81,101)(H,82,102)(H,83,103)(H,84,104)(H,85,105)(H,86,106)(H,87,107)(H,88,108)(H,89,109)(H,90,110)(H,91,111)(H,92,112)(H,93,121)(H,94,122)(H,95,115)(H,96,116)(H,97,113)(H,98,114)(H,117,118)(H,119,120)(H,123,124)(H,125,126)/t51-,52-,53-,54-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Michaelis-Menten analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526943
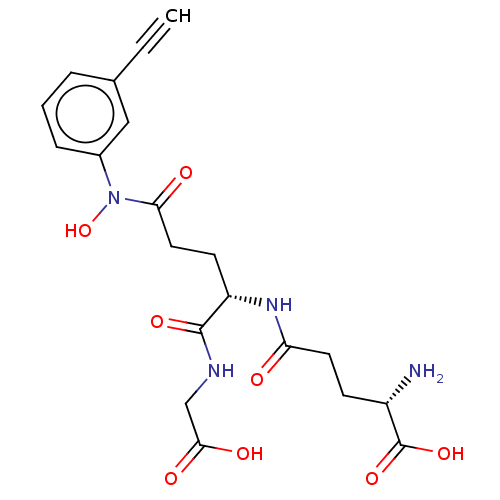
(CHEMBL4436073)Show SMILES N[C@@H](CCC(=O)N[C@@H](CCC(=O)N(O)c1cccc(c1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H24N4O8/c1-2-12-4-3-5-13(10-12)24(32)17(26)9-7-15(19(29)22-11-18(27)28)23-16(25)8-6-14(21)20(30)31/h1,3-5,10,14-15,32H,6-9,11,21H2,(H,22,29)(H,23,25)(H,27,28)(H,30,31)/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126961
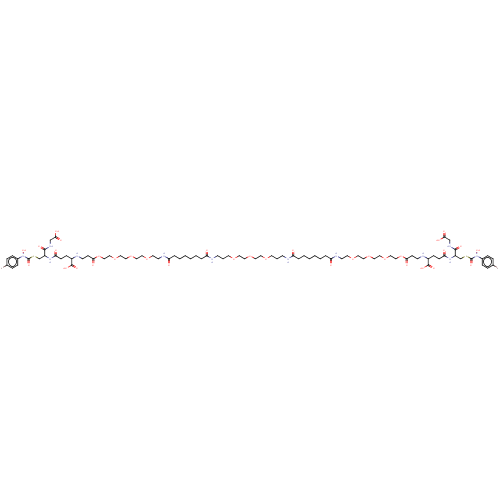
(CHEMBL3629115)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCOCCOCCOCCNC(=O)CCCCCCC(=O)NCCCOCCOCCOCCCNC(=O)CCCCCCC(=O)NCCOCCOCCOCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C82H128Br2N12O33S2/c83-59-15-19-61(20-16-59)95(117)81(115)130-57-65(77(109)91-55-73(103)104)93-71(101)25-23-63(79(111)112)85-31-27-75(107)128-53-51-126-49-47-124-45-41-121-37-33-89-69(99)13-7-3-1-5-11-67(97)87-29-9-35-119-39-43-123-44-40-120-36-10-30-88-68(98)12-6-2-4-8-14-70(100)90-34-38-122-42-46-125-48-50-127-52-54-129-76(108)28-32-86-64(80(113)114)24-26-72(102)94-66(78(110)92-56-74(105)106)58-131-82(116)96(118)62-21-17-60(84)18-22-62/h15-22,63-66,85-86,117-118H,1-14,23-58H2,(H,87,97)(H,88,98)(H,89,99)(H,90,100)(H,91,109)(H,92,110)(H,93,101)(H,94,102)(H,103,104)(H,105,106)(H,111,112)(H,113,114) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of 6-His tagged recombinant human glyoxalase 1 transfected in Escherichia coli BL21 (DE3) assessed as S-D-lactoylglutathione formation by ... |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM16452

((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...)Show SMILES OC(=O)Cc1nn(Cc2nc3cc(ccc3s2)C(F)(F)F)c(=O)c2ccccc12 Show InChI InChI=1S/C19H12F3N3O3S/c20-19(21,22)10-5-6-15-14(7-10)23-16(29-15)9-25-18(28)12-4-2-1-3-11(12)13(24-25)8-17(26)27/h1-7H,8-9H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50140172

(CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...)Show SMILES COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 using GSH and MGO as substrate by Dixon plot analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526944
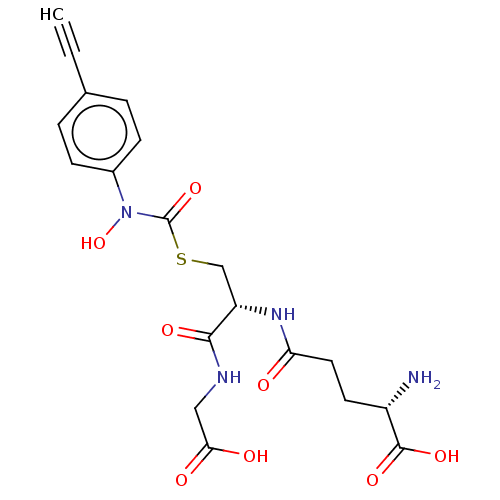
(CHEMBL4450158)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(cc1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H22N4O8S/c1-2-11-3-5-12(6-4-11)23(31)19(30)32-10-14(17(27)21-9-16(25)26)22-15(24)8-7-13(20)18(28)29/h1,3-6,13-14,31H,7-10,20H2,(H,21,27)(H,22,24)(H,25,26)(H,28,29)/t13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526942
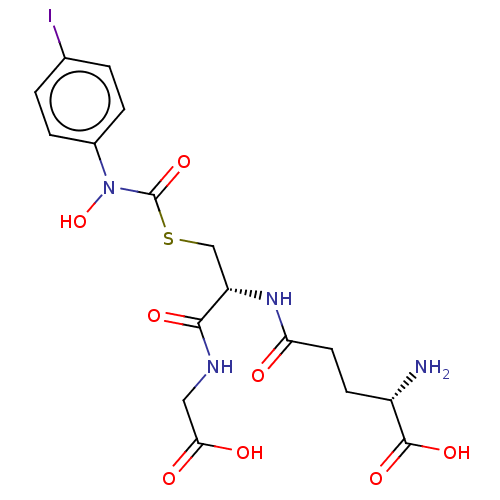
(CHEMBL4438930)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(I)cc1)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C17H21IN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092826
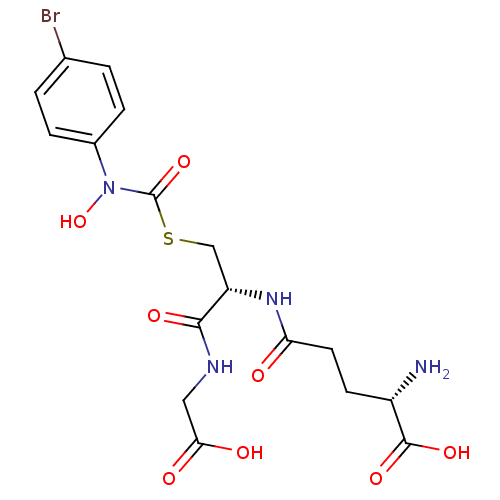
((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21BrN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092826

((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21BrN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50526948
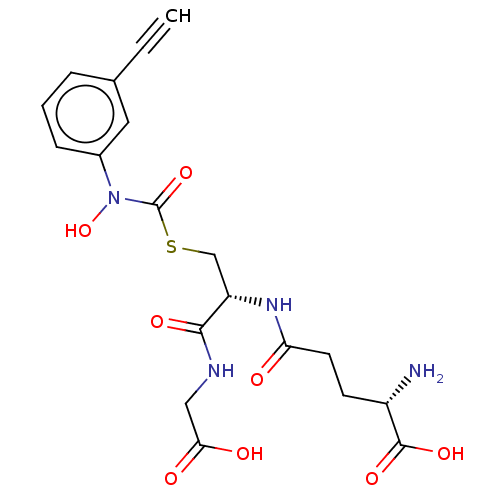
(CHEMBL4559486)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1cccc(c1)C#C)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H22N4O8S/c1-2-11-4-3-5-12(8-11)23(31)19(30)32-10-14(17(27)21-9-16(25)26)22-15(24)7-6-13(20)18(28)29/h1,3-5,8,13-14,31H,6-7,9-10,20H2,(H,21,27)(H,22,24)(H,25,26)(H,28,29)/t13-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Glyoxalase-1 using GSH and MGO as substrate |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092824
(CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21ClN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092824

(CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21ClN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126957
(CHEMBL3629119)Show SMILES CCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C22H29ClN4O10S/c1-2-37-19(31)9-10-24-15(21(33)34)7-8-17(28)26-16(20(32)25-11-18(29)30)12-38-22(35)27(36)14-5-3-13(23)4-6-14/h3-6,15-16,24,36H,2,7-12H2,1H3,(H,25,32)(H,26,28)(H,29,30)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Hydroxyacylglutathione hydrolase, mitochondrial
(Bos taurus) | BDBM50126960

(CHEMBL3629116)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCNC(=O)CCNC(=O)CC(=O)NCCNC(=O)CC(=O)NCCC(=O)NCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C58H78Br2N14O26S2/c59-33-1-5-35(6-2-33)73(97)57(95)101-31-39(53(89)69-29-49(83)84)71-43(77)11-9-37(55(91)92)61-19-15-51(87)99-25-23-67-41(75)13-17-63-45(79)27-47(81)65-21-22-66-48(82)28-46(80)64-18-14-42(76)68-24-26-100-52(88)16-20-62-38(56(93)94)10-12-44(78)72-40(54(90)70-30-50(85)86)32-102-58(96)74(98)36-7-3-34(60)4-8-36/h1-8,37-40,61-62,97-98H,9-32H2,(H,63,79)(H,64,80)(H,65,81)(H,66,82)(H,67,75)(H,68,76)(H,69,89)(H,70,90)(H,71,77)(H,72,78)(H,83,84)(H,85,86)(H,91,92)(H,93,94) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver glyoxalase 2 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126958
(CHEMBL3629118)Show SMILES CC(=O)CNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C20H25ClN4O9S/c1-11(26)8-22-14(19(31)32)6-7-16(27)24-15(18(30)23-9-17(28)29)10-35-20(33)25(34)13-4-2-12(21)3-5-13/h2-5,14-15,22,34H,6-10H2,1H3,(H,23,30)(H,24,27)(H,28,29)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Hydroxyacylglutathione hydrolase, mitochondrial
(Bos taurus) | BDBM50126961
(CHEMBL3629115)Show SMILES ON(C(=O)SCC(NC(=O)CCC(NCCC(=O)OCCOCCOCCOCCNC(=O)CCCCCCC(=O)NCCCOCCOCCOCCCNC(=O)CCCCCCC(=O)NCCOCCOCCOCCOC(=O)CCNC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O)C(O)=O)C(=O)NCC(O)=O)c1ccc(Br)cc1 Show InChI InChI=1S/C82H128Br2N12O33S2/c83-59-15-19-61(20-16-59)95(117)81(115)130-57-65(77(109)91-55-73(103)104)93-71(101)25-23-63(79(111)112)85-31-27-75(107)128-53-51-126-49-47-124-45-41-121-37-33-89-69(99)13-7-3-1-5-11-67(97)87-29-9-35-119-39-43-123-44-40-120-36-10-30-88-68(98)12-6-2-4-8-14-70(100)90-34-38-122-42-46-125-48-50-127-52-54-129-76(108)28-32-86-64(80(113)114)24-26-72(102)94-66(78(110)92-56-74(105)106)58-131-82(116)96(118)62-21-17-60(84)18-22-62/h15-22,63-66,85-86,117-118H,1-14,23-58H2,(H,87,97)(H,88,98)(H,89,99)(H,90,100)(H,91,109)(H,92,110)(H,93,101)(H,94,102)(H,103,104)(H,105,106)(H,111,112)(H,113,114) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver glyoxalase 2 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Mus musculus) | BDBM50526947
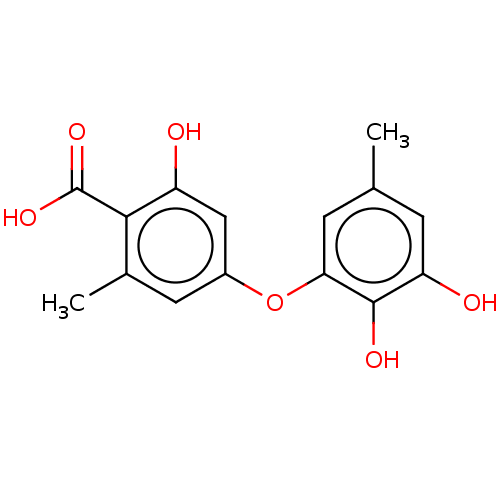
(CHEMBL4584432)Show InChI InChI=1S/C15H14O6/c1-7-3-11(17)14(18)12(4-7)21-9-5-8(2)13(15(19)20)10(16)6-9/h3-6,16-18H,1-2H3,(H,19,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant mouse His-tagged Glyoxalase-1 using GSH and MGO as substrate by spectrophotometric method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
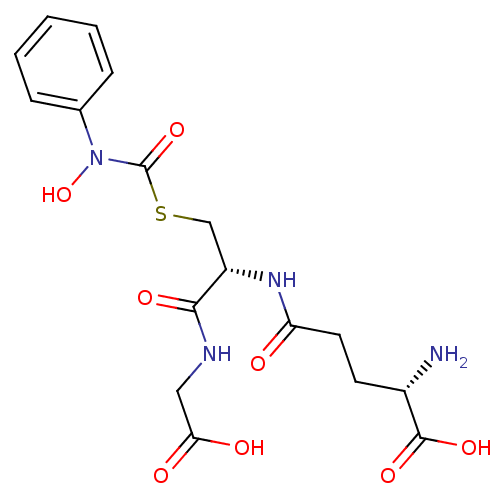
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50092825
(CHEMBL128935 | S-(N-phenyl-N-hydroxycarbamoyl)glut...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccccc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H22N4O8S/c18-11(16(26)27)6-7-13(22)20-12(15(25)19-8-14(23)24)9-30-17(28)21(29)10-4-2-1-3-5-10/h1-5,11-12,29H,6-9,18H2,(H,19,25)(H,20,22)(H,23,24)(H,26,27)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte Glyoxalase-1 |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50241121
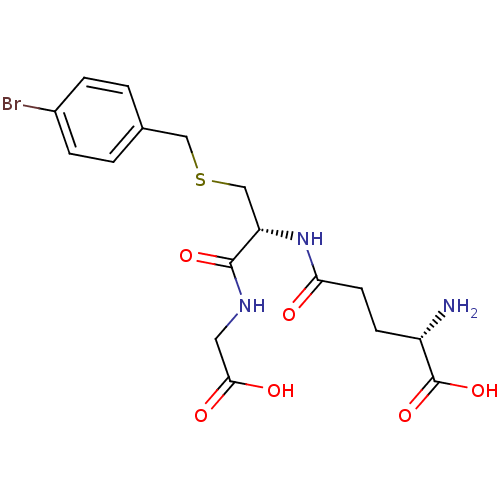
((S)-5-((R)-3-(4-bromobenzylthio)-1-(carboxymethyla...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSCc1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C17H22BrN3O6S/c18-11-3-1-10(2-4-11)8-28-9-13(16(25)20-7-15(23)24)21-14(22)6-5-12(19)17(26)27/h1-4,12-13H,5-9,19H2,(H,20,25)(H,21,22)(H,23,24)(H,26,27)/t12-,13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of Glyoxalase-1 (unknown origin) using GSH and MGO as substrates preincubated with substrates for 6 mins followed by enzyme addition by sp... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | |
Lactoylglutathione lyase
(Mus musculus) | BDBM50517464
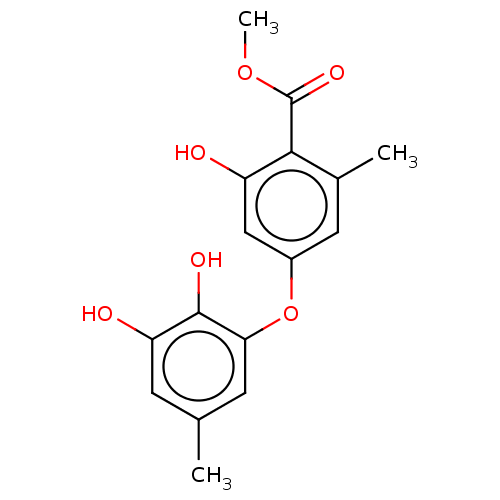
(CHEMBL1234300)Show InChI InChI=1S/C16H16O6/c1-8-4-12(18)15(19)13(5-8)22-10-6-9(2)14(11(17)7-10)16(20)21-3/h4-7,17-19H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant mouse His-tagged Glyoxalase-1 using GSH and MGO as substrate by spectrophotometric method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lactoylglutathione lyase
(Mus musculus) | BDBM50233538

(18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...)Show SMILES CC1(C)[C@@H](O)CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O |r,t:19| Show InChI InChI=1S/C30H46O4/c1-25(2)21-8-11-30(7)23(28(21,5)10-9-22(25)32)20(31)16-18-19-17-27(4,24(33)34)13-12-26(19,3)14-15-29(18,30)6/h16,19,21-23,32H,8-15,17H2,1-7H3,(H,33,34)/t19-,21-,22-,23+,26+,27-,28-,29+,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse Glyoxalase-1 expressed in Escherichia coli BL21 (DE3) pLysS cells using GSH and MGO as substrate by Dixon plot |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115243
BindingDB Entry DOI: 10.7270/Q2Z60SG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lactoylglutathione lyase
(Homo sapiens (Human)) | BDBM50126959
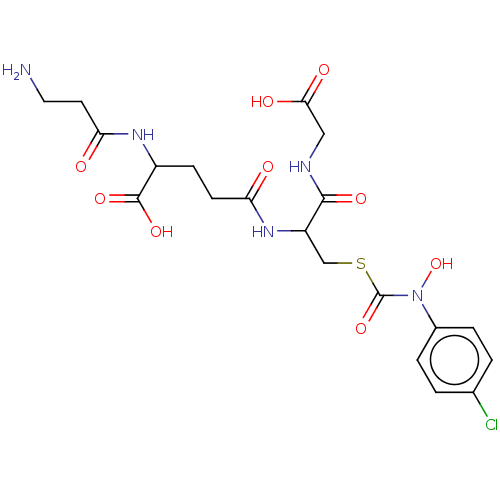
(CHEMBL3629117)Show SMILES NCCC(=O)NC(CCC(=O)NC(CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C20H26ClN5O9S/c21-11-1-3-12(4-2-11)26(35)20(34)36-10-14(18(31)23-9-17(29)30)25-15(27)6-5-13(19(32)33)24-16(28)7-8-22/h1-4,13-14,35H,5-10,22H2,(H,23,31)(H,24,28)(H,25,27)(H,29,30)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of human glyoxalase 1 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Hydroxyacylglutathione hydrolase, mitochondrial
(Bos taurus) | BDBM50092824

(CHEMBL131578 | S-(N-4chlorophenyl-N-hydroxycarbamo...)Show SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O Show InChI InChI=1S/C17H21ClN4O8S/c18-9-1-3-10(4-2-9)22(30)17(29)31-8-12(15(26)20-7-14(24)25)21-13(23)6-5-11(19)16(27)28/h1-4,11-12,30H,5-8,19H2,(H,20,26)(H,21,23)(H,24,25)(H,27,28)/t11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver glyoxalase 2 |
Bioorg Med Chem Lett 25: 4724-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.055
BindingDB Entry DOI: 10.7270/Q26H4K7B |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055889

(CHEMBL3317913)Show SMILES O=C(N1CCN(CC1)C(=O)c1cccs1)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C23H20N4O3S2/c28-21-18-5-2-1-4-17(18)19(24-25-21)13-16-12-15(14-32-16)22(29)26-7-9-27(10-8-26)23(30)20-6-3-11-31-20/h1-6,11-12,14H,7-10,13H2,(H,25,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055890

(CHEMBL3317912)Show SMILES O=C(N1CCN(CC1)C(=O)c1ccc2OCOc2c1)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C26H22N4O5S/c31-24-20-4-2-1-3-19(20)21(27-28-24)13-18-11-17(14-36-18)26(33)30-9-7-29(8-10-30)25(32)16-5-6-22-23(12-16)35-15-34-22/h1-6,11-12,14H,7-10,13,15H2,(H,28,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055887
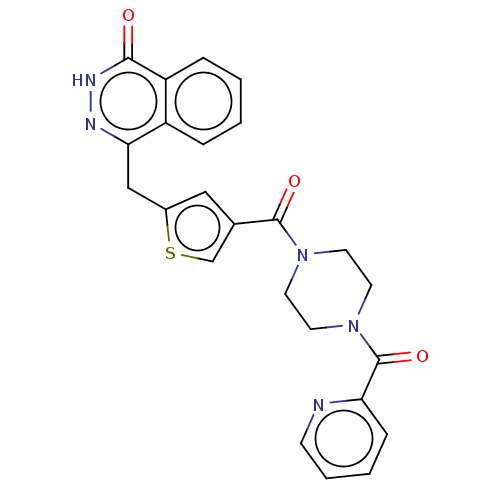
(CHEMBL3317917)Show SMILES O=C(N1CCN(CC1)C(=O)c1ccccn1)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C24H21N5O3S/c30-22-19-6-2-1-5-18(19)21(26-27-22)14-17-13-16(15-33-17)23(31)28-9-11-29(12-10-28)24(32)20-7-3-4-8-25-20/h1-8,13,15H,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055891

(CHEMBL3317909)Show SMILES Brc1ccc(cc1)C(=O)N1CCN(CC1)C(=O)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C25H21BrN4O3S/c26-18-7-5-16(6-8-18)24(32)29-9-11-30(12-10-29)25(33)17-13-19(34-15-17)14-22-20-3-1-2-4-21(20)23(31)28-27-22/h1-8,13,15H,9-12,14H2,(H,28,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055884

(CHEMBL3317900)Show SMILES CCC(=O)N1CCN(CC1)C(=O)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C21H22N4O3S/c1-2-19(26)24-7-9-25(10-8-24)21(28)14-11-15(29-13-14)12-18-16-5-3-4-6-17(16)20(27)23-22-18/h3-6,11,13H,2,7-10,12H2,1H3,(H,23,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055886
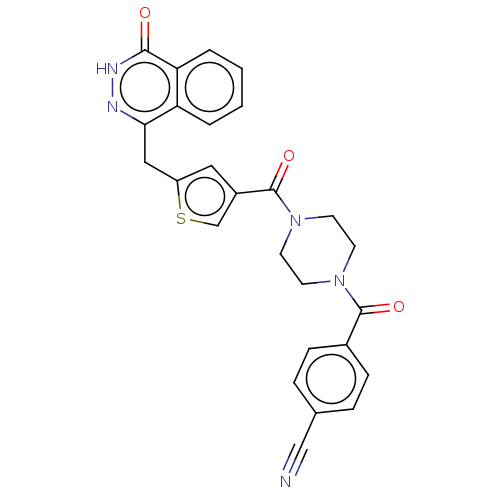
(CHEMBL3317906)Show SMILES O=C(N1CCN(CC1)C(=O)c1ccc(cc1)C#N)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C26H21N5O3S/c27-15-17-5-7-18(8-6-17)25(33)30-9-11-31(12-10-30)26(34)19-13-20(35-16-19)14-23-21-3-1-2-4-22(21)24(32)29-28-23/h1-8,13,16H,9-12,14H2,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055885
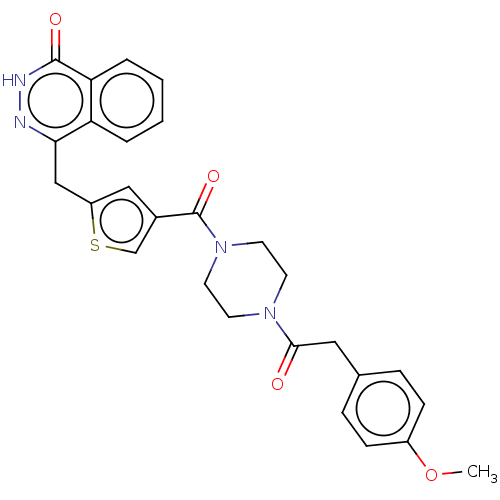
(CHEMBL3317902)Show SMILES COc1ccc(CC(=O)N2CCN(CC2)C(=O)c2csc(Cc3n[nH]c(=O)c4ccccc34)c2)cc1 Show InChI InChI=1S/C27H26N4O4S/c1-35-20-8-6-18(7-9-20)14-25(32)30-10-12-31(13-11-30)27(34)19-15-21(36-17-19)16-24-22-4-2-3-5-23(22)26(33)29-28-24/h2-9,15,17H,10-14,16H2,1H3,(H,29,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055865

(CHEMBL3317897)Show SMILES O=C(C1CC1)N1CCN(CC1)C(=O)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C22H22N4O3S/c27-20-18-4-2-1-3-17(18)19(23-24-20)12-16-11-15(13-30-16)22(29)26-9-7-25(8-10-26)21(28)14-5-6-14/h1-4,11,13-14H,5-10,12H2,(H,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055888
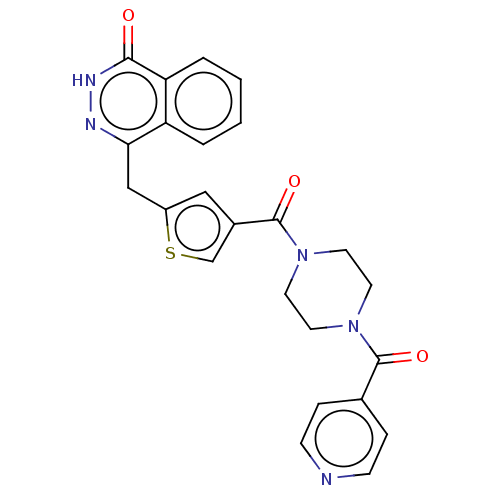
(CHEMBL3317916)Show SMILES O=C(N1CCN(CC1)C(=O)c1ccncc1)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C24H21N5O3S/c30-22-20-4-2-1-3-19(20)21(26-27-22)14-18-13-17(15-33-18)24(32)29-11-9-28(10-12-29)23(31)16-5-7-25-8-6-16/h1-8,13,15H,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50055883

(CHEMBL3317899)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1csc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C20H20N4O3S/c1-13(25)23-6-8-24(9-7-23)20(27)14-10-15(28-12-14)11-18-16-4-2-3-5-17(16)19(26)22-21-18/h2-5,10,12H,6-9,11H2,1H3,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Pharmacology and Toxicology
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
Bioorg Med Chem Lett 24: 3739-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.001
BindingDB Entry DOI: 10.7270/Q2125VBB |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM598905
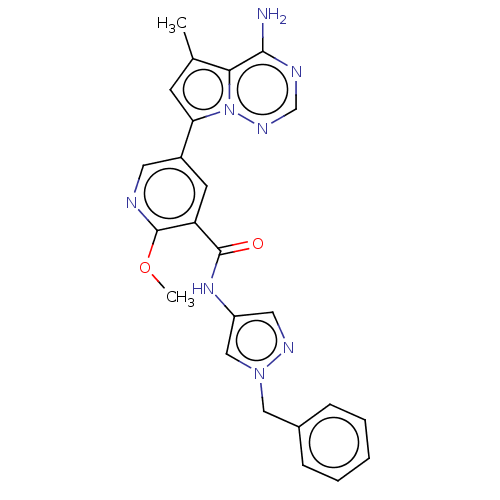
(US11618753, Example 42)Show SMILES COc1ncc(cc1C(=O)Nc1cnn(Cc2ccccc2)c1)-c1cc(C)c2c(N)ncnn12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM600172
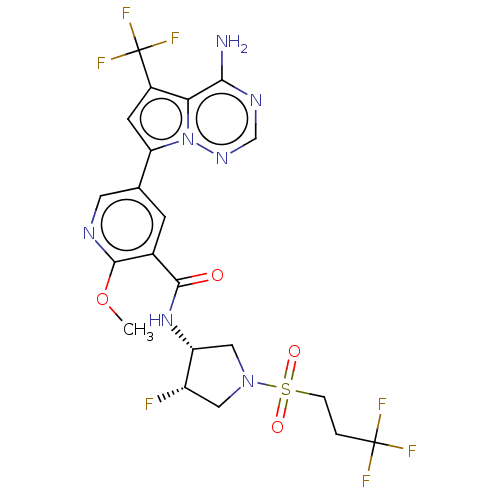
(5-[4-amino-5- (trifluoromethyl)pyrrolo [2,1-f][1,2...)Show SMILES COc1ncc(cc1C(=O)N[C@@H]1CN(C[C@@H]1F)S(=O)(=O)CCC(F)(F)F)-c1cc(c2c(N)ncnn12)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
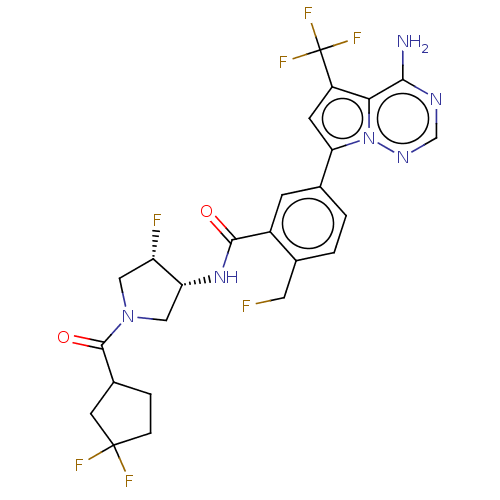
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM599722
(5-[4-amino-5- (trifluoromethyl)pyrrolo[2,1- f][1,2...)Show SMILES Nc1ncnn2c(cc(c12)C(F)(F)F)-c1ccc(CF)c(c1)C(=O)N[C@@H]1CN(C[C@@H]1F)C(=O)C1CCC(F)(F)C1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM600270
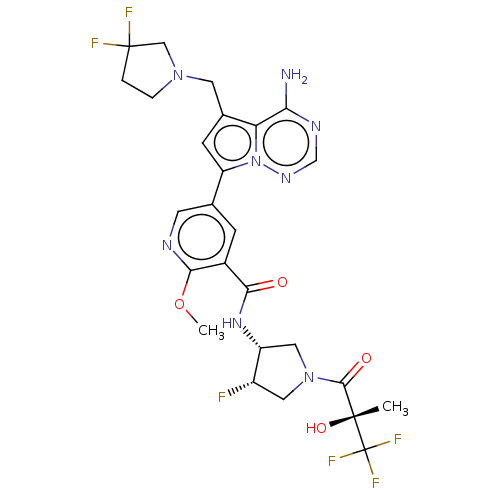
(5-{4-amino-5-[(3,3-difluoropyrrolidin-1- yl)methyl...)Show SMILES COc1ncc(cc1C(=O)N[C@@H]1CN(C[C@@H]1F)C(=O)[C@@](C)(O)C(F)(F)F)-c1cc(CN2CCC(F)(F)C2)c2c(N)ncnn12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM599068
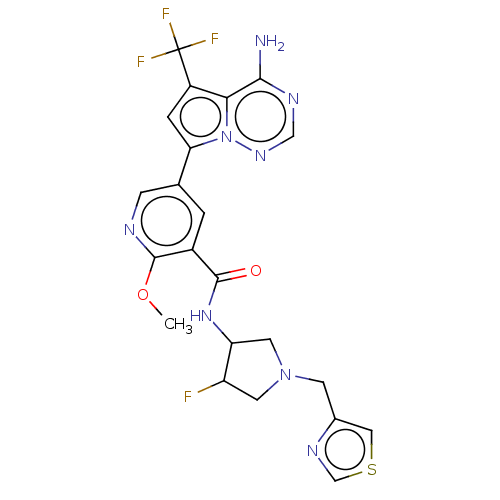
(5-[4-amino-5- (trifluoromethyl) pyrrolo[2,1- f][1,...)Show SMILES COc1ncc(cc1C(=O)NC1CN(Cc2cscn2)CC1F)-c1cc(c2c(N)ncnn12)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM599808
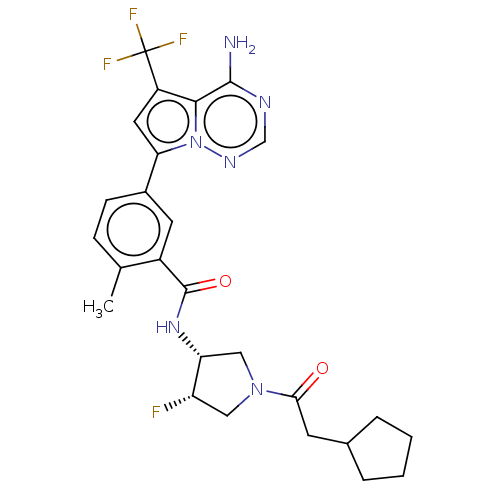
(5-[4-amino-5- (trifluoromethyl)pyrrolo[2,1- f][1,2...)Show SMILES Cc1ccc(cc1C(=O)N[C@@H]1CN(C[C@@H]1F)C(=O)CC1CCCC1)-c1cc(c2c(N)ncnn12)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM600300
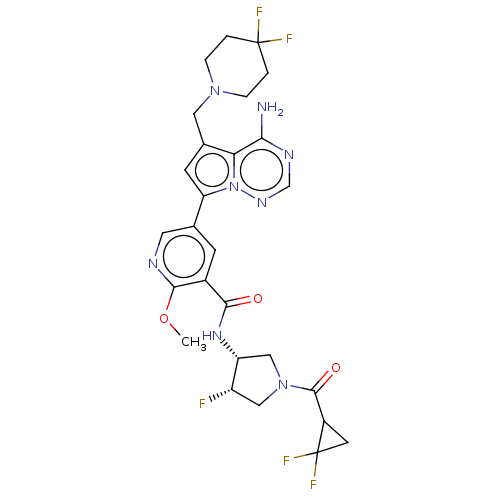
(5-{4-amino-5-[(4,4-difluoropiperidin-1- yl)methyl]...)Show SMILES COc1ncc(cc1C(=O)N[C@@H]1CN(C[C@@H]1F)C(=O)C1CC1(F)F)-c1cc(CN2CCC(F)(F)CC2)c2c(N)ncnn12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM600138
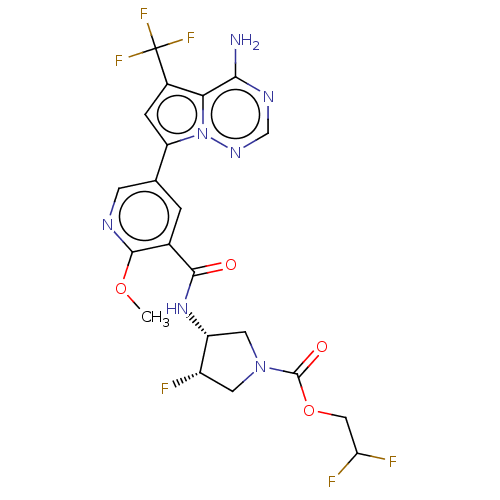
(2,2-difluoroethyl (3R,4S)-3-{5-[4-amino- 5- (trifl...)Show SMILES COc1ncc(cc1C(=O)N[C@@H]1CN(C[C@@H]1F)C(=O)OCC(F)F)-c1cc(c2c(N)ncnn12)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM599198
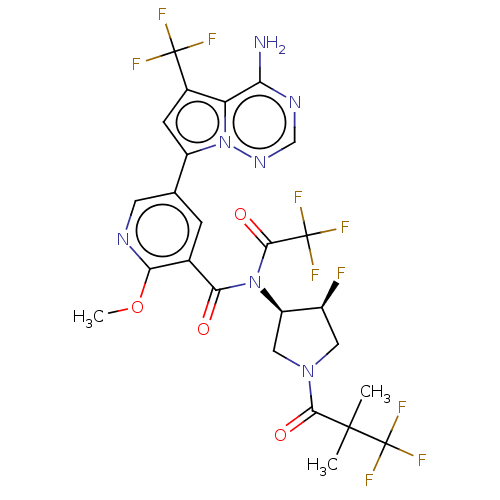
(5-[4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][1,2,...)Show SMILES COc1ncc(cc1C(=O)N([C@@H]1CN(C[C@@H]1F)C(=O)C(C)(C)C(F)(F)F)C(=O)C(F)(F)F)-c1cc(c2c(N)ncnn12)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM600178
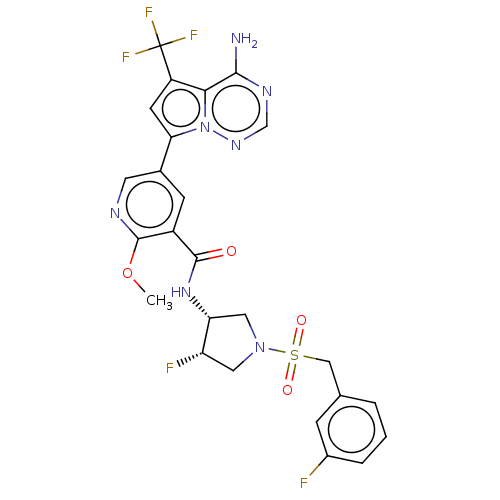
(5-[4-amino-5- (trifluoromethyl)pyrrolo [2,1-f][1,2...)Show SMILES COc1ncc(cc1C(=O)N[C@@H]1CN(C[C@@H]1F)S(=O)(=O)Cc1cccc(F)c1)-c1cc(c2c(N)ncnn12)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM598916
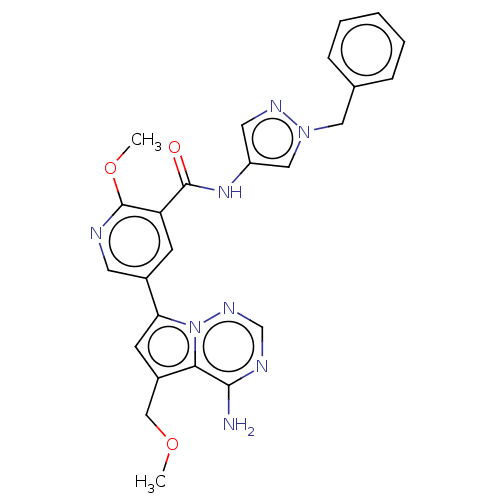
(US11618753, Example 49)Show SMILES COCc1cc(-c2cnc(OC)c(c2)C(=O)Nc2cnn(Cc3ccccc3)c2)n2ncnc(N)c12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM600177
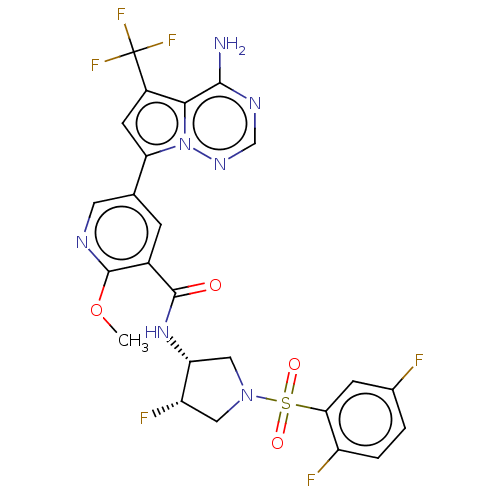
(5-[4-amino-5- (trifluoromethyl)pyrrolo [2,1-f][1,2...)Show SMILES COc1ncc(cc1C(=O)N[C@@H]1CN(C[C@@H]1F)S(=O)(=O)c1cc(F)ccc1F)-c1cc(c2c(N)ncnn12)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM600160

(5-[4-amino-5- (trifluoromethyl)pyrrolo [2,1-f][1,2...)Show SMILES COc1ncc(cc1C(=O)N[C@@H]1CCN(C1)S(=O)(=O)Cc1ccc(F)cc1)-c1cc(c2c(N)ncnn12)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM600186
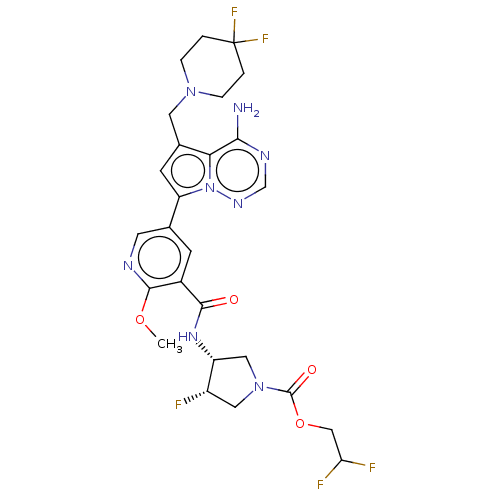
(2,2-difluoroethyl (3R,4S)-3-(5-{4-amino- 5-[(4,4- ...)Show SMILES COc1ncc(cc1C(=O)N[C@@H]1CN(C[C@@H]1F)C(=O)OCC(F)F)-c1cc(CN2CCC(F)(F)CC2)c2c(N)ncnn12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM598937

(3-[4-amino-5- (trifluoromethyl) pyrrolo[2,1- f][1,...)Show SMILES Nc1ncnn2c(cc(c12)C(F)(F)F)-c1cccc(c1)C(=O)NCC[C@@H](O)c1ccc(Cl)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A solution was prepared containing 0.2 nM Anti GST-Tb (Cisbio, 61GSTTLB), 90.6 nM probe and 1 nM His-GST-TVMV-hRIPK1(1-324) in FRET Buffer (20 mM HEP... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QF8XS4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data