

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826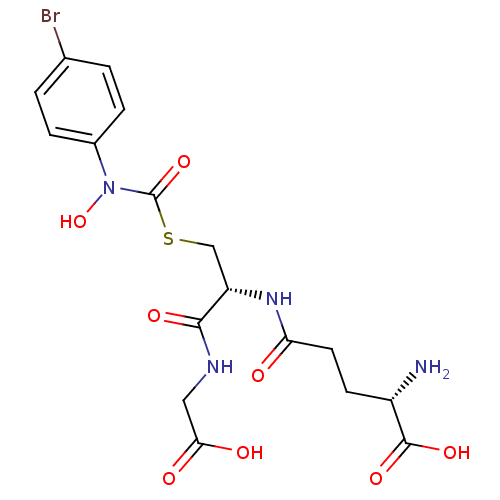 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human glyoxalase 1 | Bioorg Med Chem Lett 25: 4724-7 (2015) Article DOI: 10.1016/j.bmcl.2015.08.055 BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glyoxalase-1 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 BindingDB Entry DOI: 10.7270/Q2Z60SG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on yeast glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||