| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Urokinase-type plasminogen activator |
|---|
| Ligand | BDBM50088982 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_212977 |
|---|
| IC50 | 3.1±n/a nM |
|---|
| Citation |  Tamura, SY; Weinhouse, MI; Roberts, CA; Goldman, EA; Masukawa, K; Anderson, SM; Cohen, CR; Bradbury, AE; Bernardino, VT; Dixon, SA; Ma, MG; Nolan, TG; Brunck, TK Synthesis and biological activity of peptidyl aldehyde urokinase inhibitors. Bioorg Med Chem Lett10:983-7 (2000) [PubMed] Tamura, SY; Weinhouse, MI; Roberts, CA; Goldman, EA; Masukawa, K; Anderson, SM; Cohen, CR; Bradbury, AE; Bernardino, VT; Dixon, SA; Ma, MG; Nolan, TG; Brunck, TK Synthesis and biological activity of peptidyl aldehyde urokinase inhibitors. Bioorg Med Chem Lett10:983-7 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Urokinase-type plasminogen activator |
|---|
| Name: | Urokinase-type plasminogen activator |
|---|
| Synonyms: | 3.4.21.73 | PLAU | U-plasminogen activator | UROK_HUMAN | Urokinase | Urokinase-type plasminogen activator (uPA) | Urokinase-type plasminogen activator chain B | Urokinase-type plasminogen activator long chain A | Urokinase-type plasminogen activator short chain A | Urokinase-type plasminogen activator/surface receptor | uPA |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48528.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00749 |
|---|
| Residue: | 431 |
|---|
| Sequence: | MRALLARLLLCVLVVSDSKGSNELHQVPSNCDCLNGGTCVSNKYFSNIHWCNCPKKFGGQ
HCEIDKSKTCYEGNGHFYRGKASTDTMGRPCLPWNSATVLQQTYHAHRSDALQLGLGKHN
YCRNPDNRRRPWCYVQVGLKLLVQECMVHDCADGKKPSSPPEELKFQCGQKTLRPRFKII
GGEFTTIENQPWFAAIYRRHRGGSVTYVCGGSLISPCWVISATHCFIDYPKKEDYIVYLG
RSRLNSNTQGEMKFEVENLILHKDYSADTLAHHNDIALLKIRSKEGRCAQPSRTIQTICL
PSMYNDPQFGTSCEITGFGKENSTDYLYPEQLKMTVVKLISHRECQQPHYYGSEVTTKML
CAADPQWKTDSCQGDSGGPLVCSLQGRMTLTGIVSWGRGCALKDKPGVYTRVSHFLPWIR
SHTKEENGLAL
|
|
|
|---|
| BDBM50088982 |
|---|
| n/a |
|---|
| Name | BDBM50088982 |
|---|
| Synonyms: | CHEMBL160253 | CHEMBL367004 | N-[1-(1-Carbamimidoyl-2-hydroxy-piperidin-3-ylcarbamoyl)-ethyl]-3-hydroxy-2-(2-phenyl-ethanesulfonylamino)-propionamide; TFA |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H32N6O6S |
|---|
| Mol. Mass. | 484.57 |
|---|
| SMILES | C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)CCc1ccccc1)C(=O)N[C@H]1CCCN(C1O)C(N)=N |
|---|
| Structure | 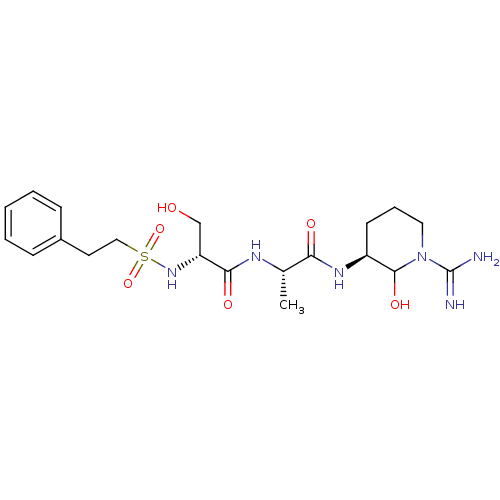
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tamura, SY; Weinhouse, MI; Roberts, CA; Goldman, EA; Masukawa, K; Anderson, SM; Cohen, CR; Bradbury, AE; Bernardino, VT; Dixon, SA; Ma, MG; Nolan, TG; Brunck, TK Synthesis and biological activity of peptidyl aldehyde urokinase inhibitors. Bioorg Med Chem Lett10:983-7 (2000) [PubMed]
Tamura, SY; Weinhouse, MI; Roberts, CA; Goldman, EA; Masukawa, K; Anderson, SM; Cohen, CR; Bradbury, AE; Bernardino, VT; Dixon, SA; Ma, MG; Nolan, TG; Brunck, TK Synthesis and biological activity of peptidyl aldehyde urokinase inhibitors. Bioorg Med Chem Lett10:983-7 (2000) [PubMed]