

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50054502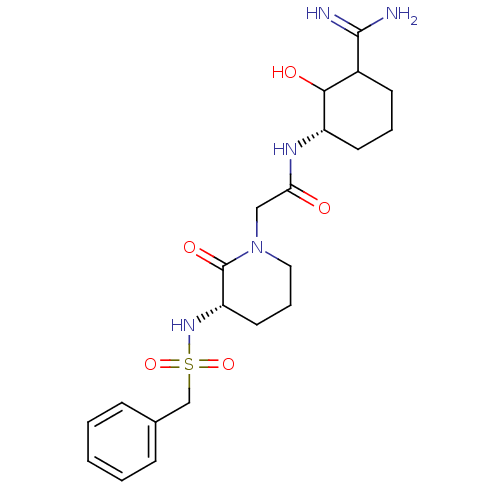 (CHEMBL140494 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description compound was tested in vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087645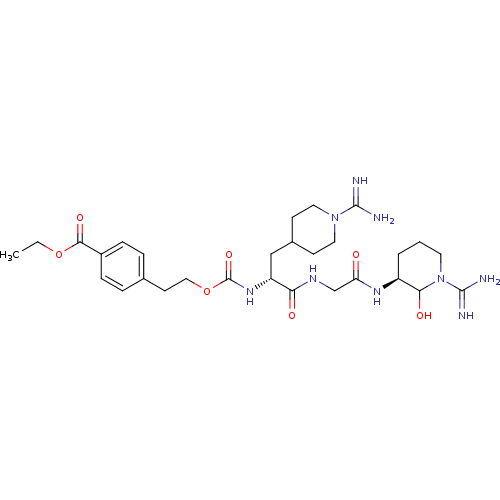 (4-{2-[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087641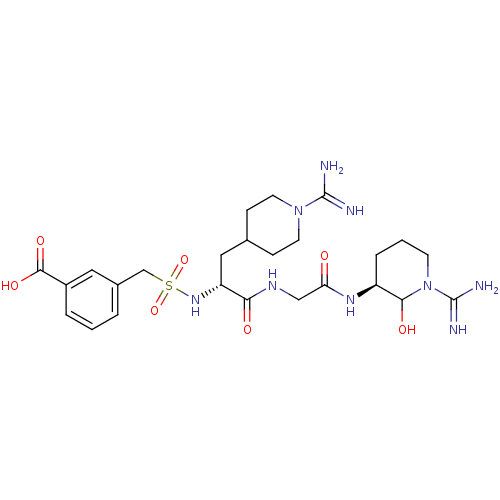 (3-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human trypsin. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087639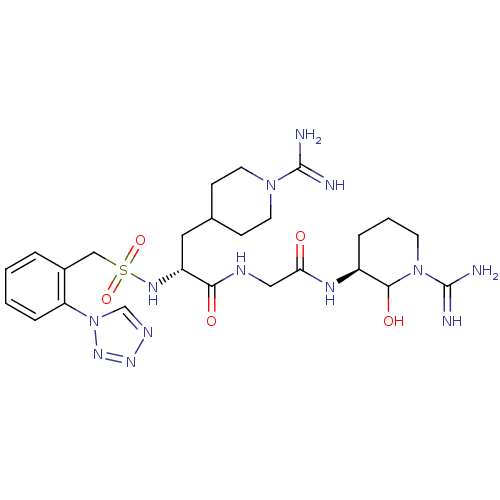 ((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration of the compound required for classical fast inhibition of cleavage of the chromogenic substrate by human enzyme Coagulation factor X in... | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087647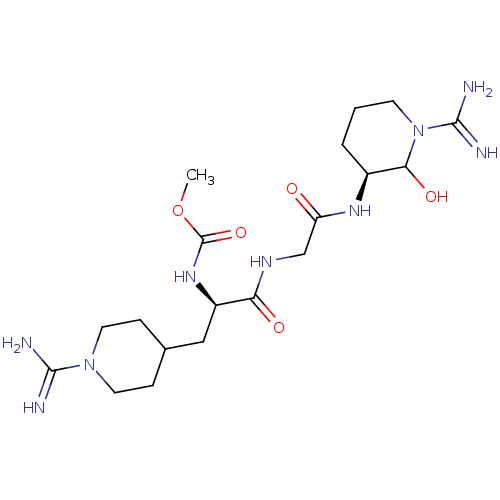 (CHEMBL162277 | [(R)-1-{[((S)-1-Carbamimidoyl-2-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Coagulation factor X | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50289560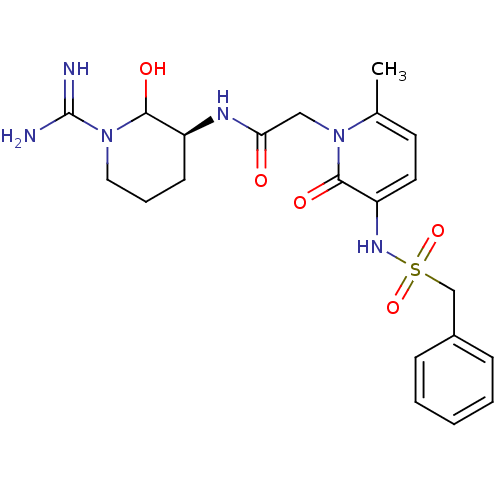 (CHEMBL289389 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.467 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required to inhibit human thrombin enzyme was determined | Bioorg Med Chem Lett 7: 1543-1548 (1997) Article DOI: 10.1016/S0960-894X(97)00258-8 BindingDB Entry DOI: 10.7270/Q2HD7VNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076074 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.505 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076074 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.505 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required to inhibit human thrombin enzyme was determined | Bioorg Med Chem Lett 7: 1543-1548 (1997) Article DOI: 10.1016/S0960-894X(97)00258-8 BindingDB Entry DOI: 10.7270/Q2HD7VNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054498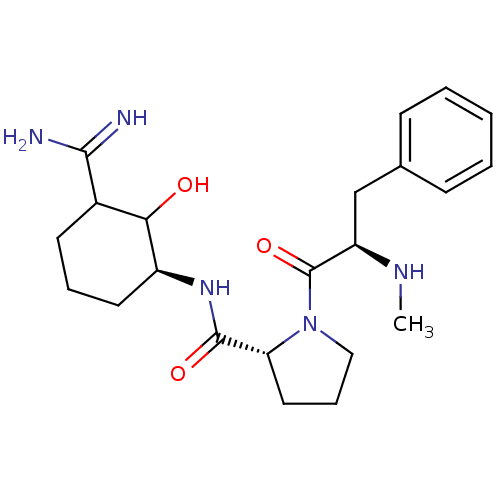 ((R)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054493 (CHEMBL343805 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073429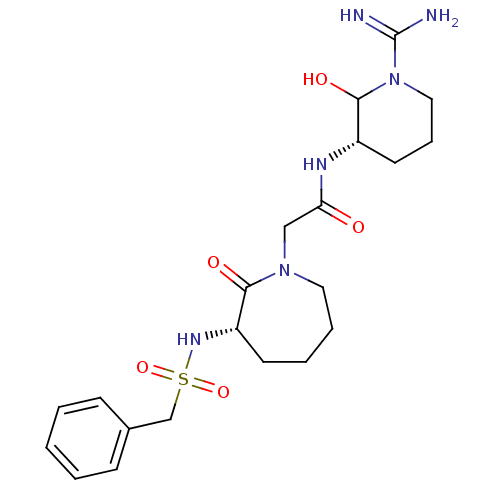 (CHEMBL82032 | CHEMBL84575 | N-((S)-1-Carbamimidoyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibitory activity against Coagulation factor II | Bioorg Med Chem Lett 7: 2421-2426 (1997) Article DOI: 10.1016/S0960-894X(97)00446-0 BindingDB Entry DOI: 10.7270/Q2X92B91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50290996 (3-tert-Butoxycarbonylamino-4-[2-(4-guanidino-1-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50% inhibitory concentration against cow protease enzyme trypsin | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087644 ((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087638 ((R)-5-Guanidino-2-phenylmethanesulfonylamino-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087635 (((R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibition of human VCAM and Ramos cell VLA-4 interaction | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087635 (((R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibition of human VCAM and Ramos cell VLA-4 interaction | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087635 (((R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087636 ((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087636 ((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibition of human VCAM and Ramos cell VLA-4 interaction | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087643 (2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054492 ((S)-4-[(R)-2-((S)-3-Carbamimidoyl-2-hydroxy-cycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50291004 (4-[2-(1-Carbamimidoyl-2-hydroxy-piperidin-3-ylcarb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory concentration required to inhibit human serine protease enzyme thrombin (FIIa) by 50% | Bioorg Med Chem Lett 7: 331-336 (1997) Article DOI: 10.1016/S0960-894X(97)00004-8 BindingDB Entry DOI: 10.7270/Q2Z03850 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087642 (CHEMBL163251 | N-[((S)-1-Carbamimidoyl-2-hydroxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054494 (2-({(S)-1-[((S)-3-Carbamimidoyl-2-hydroxy-cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054492 ((S)-4-[(R)-2-((S)-3-Carbamimidoyl-2-hydroxy-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087640 (2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISA | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087640 (2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50291004 (4-[2-(1-Carbamimidoyl-2-hydroxy-piperidin-3-ylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory concentration required to inhibit human serine protease enzyme human trypsin by 50% | Bioorg Med Chem Lett 7: 331-336 (1997) Article DOI: 10.1016/S0960-894X(97)00004-8 BindingDB Entry DOI: 10.7270/Q2Z03850 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50054498 ((R)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease Trypsin. | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50081158 (2-((R)-3-Amino-3-benzyl-2-oxo-piperidin-1-yl)-N-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibitory activity against Coagulation factor II | Bioorg Med Chem Lett 7: 2421-2426 (1997) Article DOI: 10.1016/S0960-894X(97)00446-0 BindingDB Entry DOI: 10.7270/Q2X92B91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087637 (2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISA | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087637 (2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087634 (3-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50228840 ((S)-1-[(R)-2-(3,3-dimethyl-butyrylamino)-3-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | Bioorg Med Chem Lett 7: 1359-1364 (1997) Article DOI: 10.1016/S0960-894X(97)00227-8 BindingDB Entry DOI: 10.7270/Q2542NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50289559 (CHEMBL39475 | N-((S)-1-Carbamimidoyl-2-hydroxy-pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required to inhibit human thrombin enzyme was determined | Bioorg Med Chem Lett 7: 1543-1548 (1997) Article DOI: 10.1016/S0960-894X(97)00258-8 BindingDB Entry DOI: 10.7270/Q2HD7VNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50290996 (3-tert-Butoxycarbonylamino-4-[2-(4-guanidino-1-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its 50 percent inhibitory concentration against human thrombin factor (FIIa) | Bioorg Med Chem Lett 7: 315-320 (1997) Article DOI: 10.1016/S0960-894X(97)00005-X BindingDB Entry DOI: 10.7270/Q22R3RNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50291006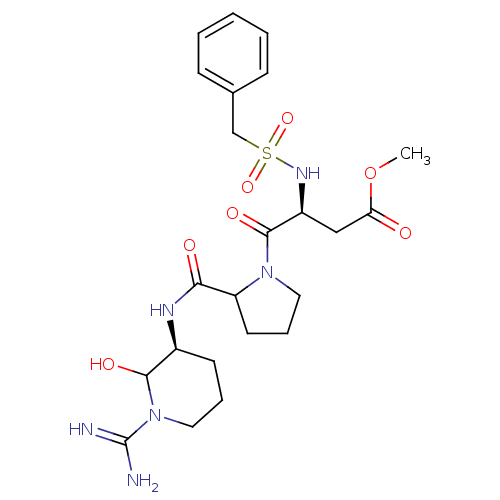 (4-[2-(1-Carbamimidoyl-2-hydroxy-piperidin-3-ylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory concentration required to inhibit human serine protease enzyme human trypsin by 50% | Bioorg Med Chem Lett 7: 331-336 (1997) Article DOI: 10.1016/S0960-894X(97)00004-8 BindingDB Entry DOI: 10.7270/Q2Z03850 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087639 ((R)-N-[((S)-1-Carbamimidoyl-2-hydroxy-piperidin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISA | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088982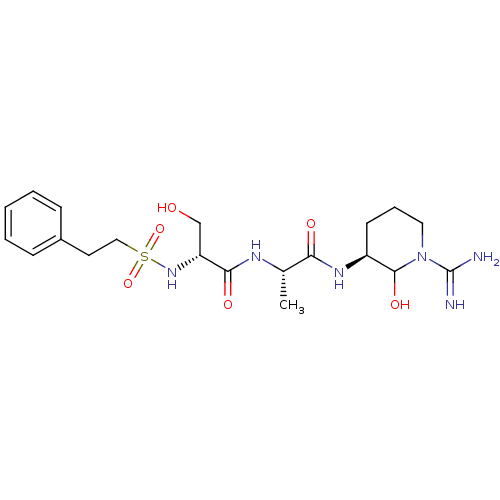 (CHEMBL160253 | CHEMBL367004 | N-[1-(1-Carbamimidoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087641 (3-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human Coagulation factor X. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50291006 (4-[2-(1-Carbamimidoyl-2-hydroxy-piperidin-3-ylcarb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory concentration required to inhibit human serine protease enzyme thrombin (FIIa) by 50% | Bioorg Med Chem Lett 7: 331-336 (1997) Article DOI: 10.1016/S0960-894X(97)00004-8 BindingDB Entry DOI: 10.7270/Q2Z03850 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088978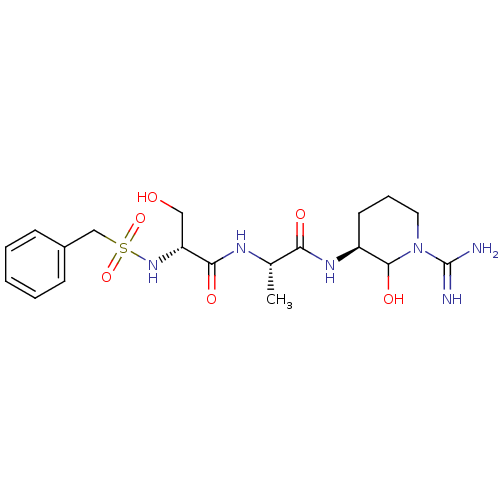 (CHEMBL176515 | N-[1-(1-Carbamimidoyl-2-hydroxy-pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087646 (2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit the cleavage of the chromogenic substrate by human enzyme Coagulation factor X in vitro | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087646 (2-{[(R)-1-{[((S)-1-Carbamimidoyl-2-hydroxy-piperid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human plasmin. | Bioorg Med Chem Lett 10: 745-9 (2000) BindingDB Entry DOI: 10.7270/Q2D50M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071693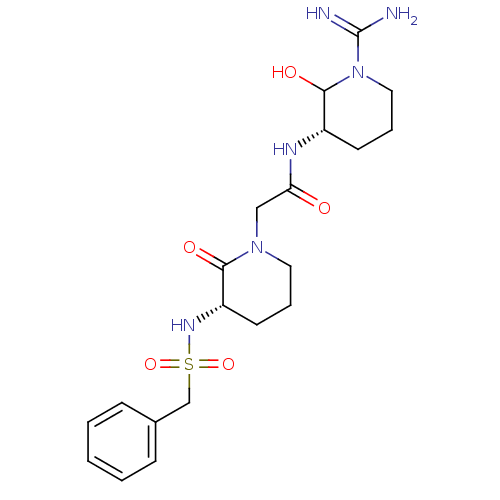 (CHEMBL38927 | CVS-1578 | N-((S)-1-Carbamimidoyl-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required to inhibit human thrombin enzyme was determined | Bioorg Med Chem Lett 7: 1543-1548 (1997) Article DOI: 10.1016/S0960-894X(97)00258-8 BindingDB Entry DOI: 10.7270/Q2HD7VNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054502 (CHEMBL140494 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071693 (CHEMBL38927 | CVS-1578 | N-((S)-1-Carbamimidoyl-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibitory concentration required to inhibit human serine protease enzyme thrombin (FIIa) by 50% | Bioorg Med Chem Lett 7: 331-336 (1997) Article DOI: 10.1016/S0960-894X(97)00004-8 BindingDB Entry DOI: 10.7270/Q2Z03850 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076073 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071693 (CHEMBL38927 | CVS-1578 | N-((S)-1-Carbamimidoyl-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibitory activity against Coagulation factor II | Bioorg Med Chem Lett 7: 2421-2426 (1997) Article DOI: 10.1016/S0960-894X(97)00446-0 BindingDB Entry DOI: 10.7270/Q2X92B91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50289436 ((S)-2-((R)-2-Acetylamino-3-phenyl-propionylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | Bioorg Med Chem Lett 7: 1359-1364 (1997) Article DOI: 10.1016/S0960-894X(97)00227-8 BindingDB Entry DOI: 10.7270/Q2542NM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 310 total ) | Next | Last >> |