| Reaction Details |
|---|
 | Report a problem with these data |
| Target | High affinity nerve growth factor receptor |
|---|
| Ligand | BDBM50108297 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_210738 |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Murakata, C; Kaneko, M; Gessner, G; Angeles, TS; Ator, MA; O'Kane, TM; McKenna, BA; Thomas, BA; Mathiasen, JR; Saporito, MS; Bozyczko-Coyne, D; Hudkins, RL Mixed lineage kinase activity of indolocarbazole analogues. Bioorg Med Chem Lett12:147-50 (2001) [PubMed] Murakata, C; Kaneko, M; Gessner, G; Angeles, TS; Ator, MA; O'Kane, TM; McKenna, BA; Thomas, BA; Mathiasen, JR; Saporito, MS; Bozyczko-Coyne, D; Hudkins, RL Mixed lineage kinase activity of indolocarbazole analogues. Bioorg Med Chem Lett12:147-50 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| High affinity nerve growth factor receptor |
|---|
| Name: | High affinity nerve growth factor receptor |
|---|
| Synonyms: | 2.7.10.1 | MTC | NTRK1 | NTRK1/NTRK2 | NTRK1_HUMAN | Nerve growth factor receptor Trk-A | Neurotrophic tyrosine kinase receptor type 1 | Neurotrophic tyrosine kinase receptor type 1 (TrkA) | Synonyms=MTC | TRK | TRK1-transforming tyrosine kinase protein | TRKA | TRKA GN | TRKA GN | Trk-A | Tropomyosin alpha-3 chain/High affinity nerve growth factor receptor | Tropomyosin-related kinase A | Tropomyosin-related kinase A (TrkA) | Tyrosine kinase receptor | Tyrosine kinase receptor (Trk) | Tyrosine kinase receptor A | Tyrosine kinase receptor A (Trk A) | Tyrosine kinase receptor A (Trk-A) | Tyrosine kinase receptor A (TrkA) | gp140trk | p140-TrkA |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 87498.18 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P04629 |
|---|
| Residue: | 796 |
|---|
| Sequence: | MLRGGRRGQLGWHSWAAGPGSLLAWLILASAGAAPCPDACCPHGSSGLRCTRDGALDSLH
HLPGAENLTELYIENQQHLQHLELRDLRGLGELRNLTIVKSGLRFVAPDAFHFTPRLSRL
NLSFNALESLSWKTVQGLSLQELVLSGNPLHCSCALRWLQRWEEEGLGGVPEQKLQCHGQ
GPLAHMPNASCGVPTLKVQVPNASVDVGDDVLLRCQVEGRGLEQAGWILTELEQSATVMK
SGGLPSLGLTLANVTSDLNRKNVTCWAENDVGRAEVSVQVNVSFPASVQLHTAVEMHHWC
IPFSVDGQPAPSLRWLFNGSVLNETSFIFTEFLEPAANETVRHGCLRLNQPTHVNNGNYT
LLAANPFGQASASIMAAFMDNPFEFNPEDPIPVSFSPVDTNSTSGDPVEKKDETPFGVSV
AVGLAVFACLFLSTLLLVLNKCGRRNKFGINRPAVLAPEDGLAMSLHFMTLGGSSLSPTE
GKGSGLQGHIIENPQYFSDACVHHIKRRDIVLKWELGEGAFGKVFLAECHNLLPEQDKML
VAVKALKEASESARQDFQREAELLTMLQHQHIVRFFGVCTEGRPLLMVFEYMRHGDLNRF
LRSHGPDAKLLAGGEDVAPGPLGLGQLLAVASQVAAGMVYLAGLHFVHRDLATRNCLVGQ
GLVVKIGDFGMSRDIYSTDYYRVGGRTMLPIRWMPPESILYRKFTTESDVWSFGVVLWEI
FTYGKQPWYQLSNTEAIDCITQGRELERPRACPPEVYAIMRGCWQREPQQRHSIKDVHAR
LQALAQAPPVYLDVLG
|
|
|
|---|
| BDBM50108297 |
|---|
| n/a |
|---|
| Name | BDBM50108297 |
|---|
| Synonyms: | CHEMBL288816 | Indolocarbazole analogue |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C35H37N3O5S2 |
|---|
| Mol. Mass. | 643.815 |
|---|
| SMILES | COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccc(CSC(C)C)cc3c3c4CNC(=O)c4c4c5cc(CSC(C)C)ccc5n2c4c13 |
|---|
| Structure | 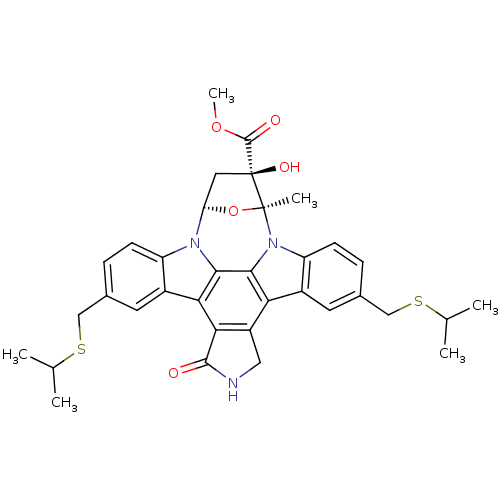
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Murakata, C; Kaneko, M; Gessner, G; Angeles, TS; Ator, MA; O'Kane, TM; McKenna, BA; Thomas, BA; Mathiasen, JR; Saporito, MS; Bozyczko-Coyne, D; Hudkins, RL Mixed lineage kinase activity of indolocarbazole analogues. Bioorg Med Chem Lett12:147-50 (2001) [PubMed]
Murakata, C; Kaneko, M; Gessner, G; Angeles, TS; Ator, MA; O'Kane, TM; McKenna, BA; Thomas, BA; Mathiasen, JR; Saporito, MS; Bozyczko-Coyne, D; Hudkins, RL Mixed lineage kinase activity of indolocarbazole analogues. Bioorg Med Chem Lett12:147-50 (2001) [PubMed]