 Found 142 hits with Last Name = 'gessner' and Initial = 'g'
Found 142 hits with Last Name = 'gessner' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A) dopamine receptor
(RAT) | BDBM60917
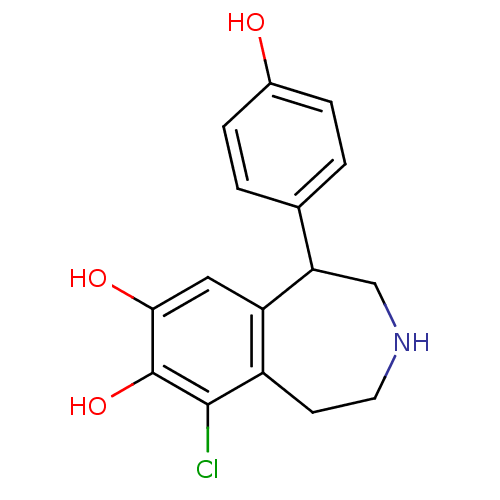
(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(RAT) | BDBM50025202

(9-Aminomethyl-9H-fluorene-2,5,6-triol | CHEMBL5767...)Show InChI InChI=1S/C14H13NO3/c15-6-11-9-3-4-12(17)14(18)13(9)8-2-1-7(16)5-10(8)11/h1-5,11,16-18H,6,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(RAT) | BDBM50025206
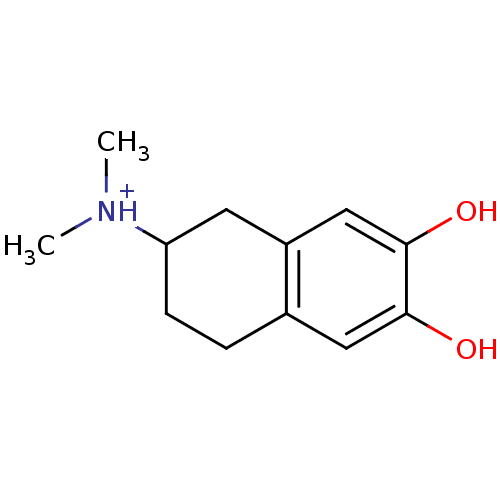
((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...)Show InChI InChI=1S/C12H17NO2/c1-13(2)10-4-3-8-6-11(14)12(15)7-9(8)5-10/h6-7,10,14-15H,3-5H2,1-2H3/p+1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(BOVINE) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50025206

((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...)Show InChI InChI=1S/C12H17NO2/c1-13(2)10-4-3-8-6-11(14)12(15)7-9(8)5-10/h6-7,10,14-15H,3-5H2,1-2H3/p+1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025201
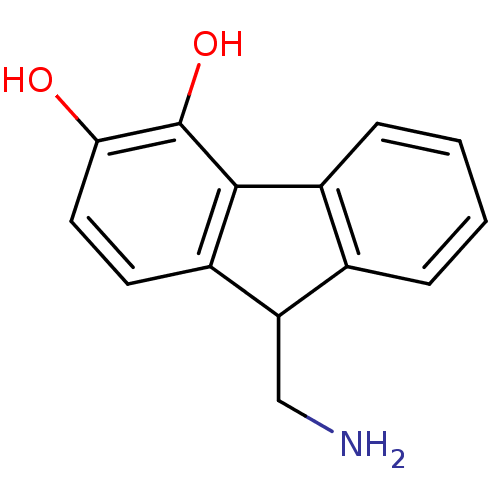
(9-Aminomethyl-9H-fluorene-3,4-diol | CHEMBL55693)Show InChI InChI=1S/C14H13NO2/c15-7-11-8-3-1-2-4-9(8)13-10(11)5-6-12(16)14(13)17/h1-6,11,16-17H,7,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025208

(6-Chloro-9-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...)Show InChI InChI=1S/C16H16ClNO2/c17-14-12-7-9-18-8-6-11(12)13(15(19)16(14)20)10-4-2-1-3-5-10/h1-5,18-20H,6-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50004821

(2,3,4,5-Tetrahydro-1H-benzo[d]azepine-7,8-diol | C...)Show InChI InChI=1S/C10H13NO2/c12-9-5-7-1-3-11-4-2-8(7)6-10(9)13/h5-6,11-13H,1-4H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against dDopamine receptor D1 using [3H]fenoldopam as a radioligand |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025204
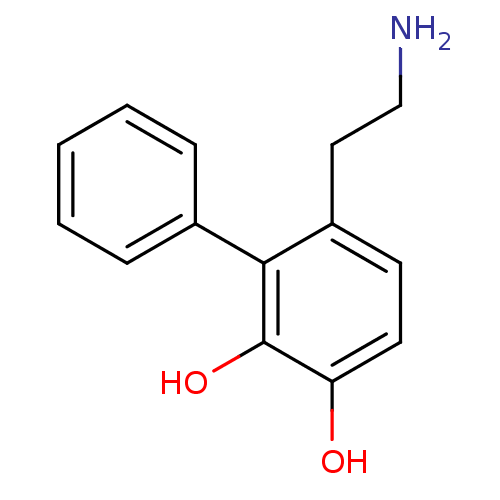
(6-(2-Amino-ethyl)-biphenyl-2,3-diol | CHEMBL299511)Show InChI InChI=1S/C14H15NO2/c15-9-8-11-6-7-12(16)14(17)13(11)10-4-2-1-3-5-10/h1-7,16-17H,8-9,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025209
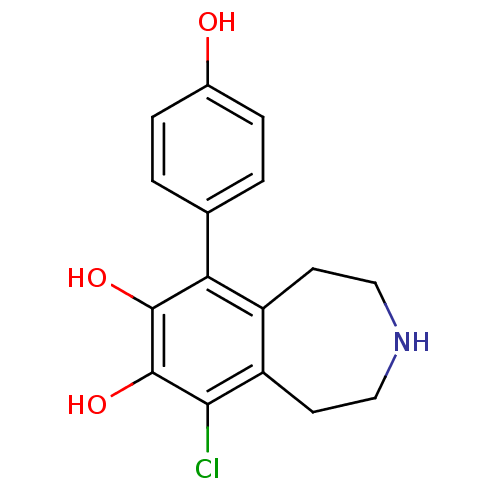
(6-Chloro-9-(4-hydroxy-phenyl)-2,3,4,5-tetrahydro-1...)Show InChI InChI=1S/C16H16ClNO3/c17-14-12-6-8-18-7-5-11(12)13(15(20)16(14)21)9-1-3-10(19)4-2-9/h1-4,18-21H,5-8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025207
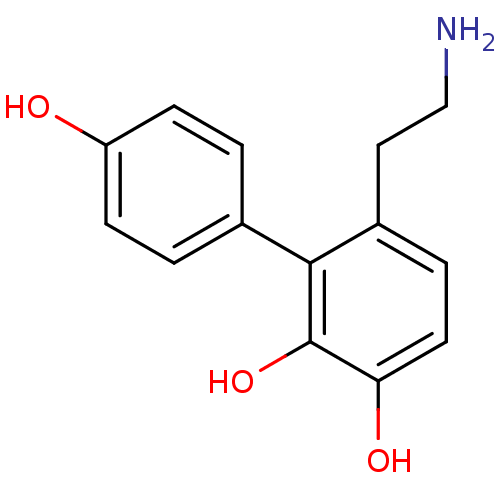
(6-(2-Amino-ethyl)-biphenyl-2,3,4'-triol | CHEMBL57...)Show InChI InChI=1S/C14H15NO3/c15-8-7-10-3-6-12(17)14(18)13(10)9-1-4-11(16)5-2-9/h1-6,16-18H,7-8,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025203
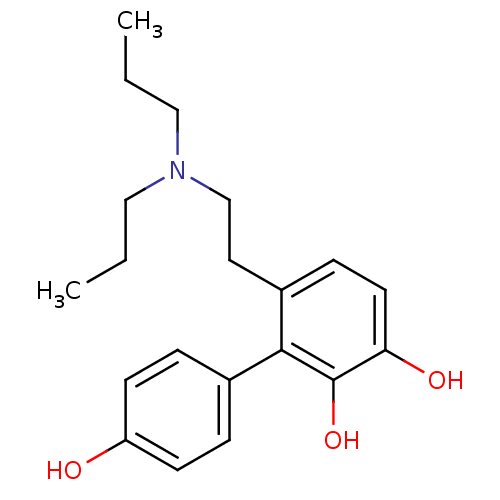
(6-(2-Dipropylamino-ethyl)-biphenyl-2,3,4'-triol | ...)Show InChI InChI=1S/C20H27NO3/c1-3-12-21(13-4-2)14-11-16-7-10-18(23)20(24)19(16)15-5-8-17(22)9-6-15/h5-10,22-24H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025210

(6-Phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine-7,8...)Show InChI InChI=1S/C16H17NO2/c18-14-10-12-6-8-17-9-7-13(12)15(16(14)19)11-4-2-1-3-5-11/h1-5,10,17-19H,6-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50025203

(6-(2-Dipropylamino-ethyl)-biphenyl-2,3,4'-triol | ...)Show InChI InChI=1S/C20H27NO3/c1-3-12-21(13-4-2)14-11-16-7-10-18(23)20(24)19(16)15-5-8-17(22)9-6-15/h5-10,22-24H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025205

(6-(2-Dipropylamino-ethyl)-biphenyl-2,3-diol | CHEM...)Show InChI InChI=1S/C20H27NO2/c1-3-13-21(14-4-2)15-12-17-10-11-18(22)20(23)19(17)16-8-6-5-7-9-16/h5-11,22-23H,3-4,12-15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50025211

(6-(4-Hydroxy-phenyl)-2,3,4,5-tetrahydro-1H-benzo[d...)Show InChI InChI=1S/C16H17NO3/c18-12-3-1-10(2-4-12)15-13-6-8-17-7-5-11(13)9-14(19)16(15)20/h1-4,9,17-20H,5-8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50025202

(9-Aminomethyl-9H-fluorene-2,5,6-triol | CHEMBL5767...)Show InChI InChI=1S/C14H13NO3/c15-6-11-9-3-4-12(17)14(18)13(9)8-2-1-7(16)5-10(8)11/h1-5,11,16-18H,6,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50118955
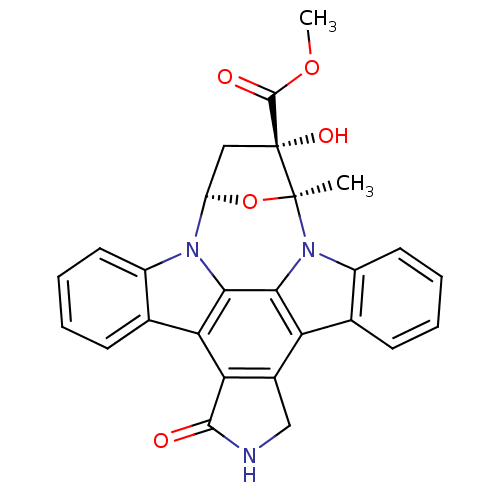
(CHEMBL94678 | Compound K-252a(epi))Show SMILES COC(=O)[C@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18-,26+,27-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of baculovirus expressed human trkA |
J Med Chem 48: 3776-83 (2005)
Article DOI: 10.1021/jm040178m
BindingDB Entry DOI: 10.7270/Q2HX1C66 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50410231

(CHEMBL2113064)Show SMILES CO[C@]1(CO)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C27H23N3O4/c1-26-27(13-31,33-2)11-19(34-26)29-17-9-5-3-7-14(17)21-22-16(12-28-25(22)32)20-15-8-4-6-10-18(15)30(26)24(20)23(21)29/h3-10,19,31H,11-13H2,1-2H3,(H,28,32)/t19-,26+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of baculovirus expressed human trkA |
J Med Chem 48: 3776-83 (2005)
Article DOI: 10.1021/jm040178m
BindingDB Entry DOI: 10.7270/Q2HX1C66 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha/beta/delta/epsilon/eta/gamma/theta/zeta type
(Rattus norvegicus-Rattus norvegicus (Rat)-Rattus n...) | BDBM50108296
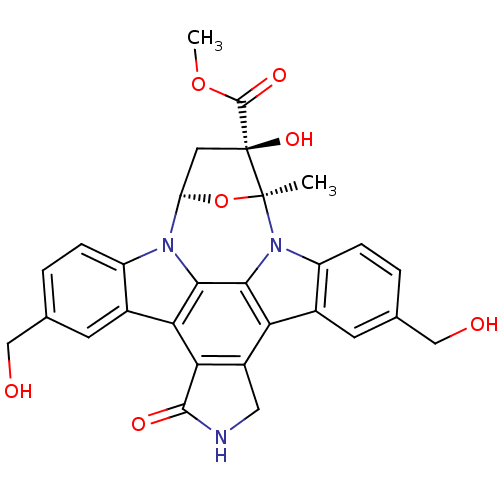
(CHEMBL289772 | Indolocarbazole analogue)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccc(CO)cc3c3c4CNC(=O)c4c4c5cc(CO)ccc5n2c4c13 Show InChI InChI=1S/C29H25N3O7/c1-28-29(37,27(36)38-2)9-20(39-28)31-18-5-3-13(11-33)7-15(18)22-23-17(10-30-26(23)35)21-16-8-14(12-34)4-6-19(16)32(28)25(21)24(22)31/h3-8,20,33-34,37H,9-12H2,1-2H3,(H,30,35)/t20?,28-,29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50410233
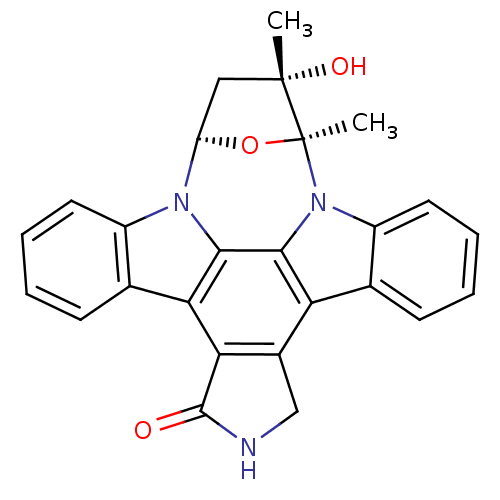
(CHEMBL2113063)Show SMILES C[C@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C26H21N3O3/c1-25(31)11-18-28-16-9-5-3-7-13(16)20-21-15(12-27-24(21)30)19-14-8-4-6-10-17(14)29(23(19)22(20)28)26(25,2)32-18/h3-10,18,31H,11-12H2,1-2H3,(H,27,30)/t18-,25+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of baculovirus expressed human trkA |
J Med Chem 48: 3776-83 (2005)
Article DOI: 10.1021/jm040178m
BindingDB Entry DOI: 10.7270/Q2HX1C66 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50167984
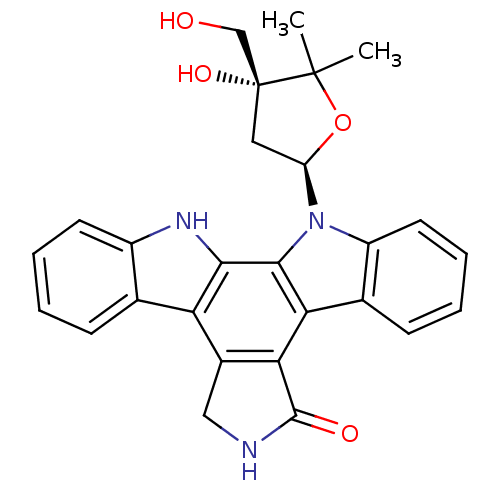
(CHEMBL370493 | K-252a analogue)Show SMILES CC1(C)O[C@H](C[C@@]1(O)CO)n1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C27H25N3O4/c1-26(2)27(33,13-31)11-19(34-26)30-18-10-6-4-8-15(18)21-22-16(12-28-25(22)32)20-14-7-3-5-9-17(14)29-23(20)24(21)30/h3-10,19,29,31,33H,11-13H2,1-2H3,(H,28,32)/t19-,27-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of baculovirus expressed human trkA |
J Med Chem 48: 3776-83 (2005)
Article DOI: 10.1021/jm040178m
BindingDB Entry DOI: 10.7270/Q2HX1C66 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50167983

(CHEMBL365585 | K-252a analogue)Show SMILES CC1(C)O[C@H](C[C@]1(O)CO)n1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C27H25N3O4/c1-26(2)27(33,13-31)11-19(34-26)30-18-10-6-4-8-15(18)21-22-16(12-28-25(22)32)20-14-7-3-5-9-17(14)29-23(20)24(21)30/h3-10,19,29,31,33H,11-13H2,1-2H3,(H,28,32)/t19-,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of baculovirus expressed human trkA |
J Med Chem 48: 3776-83 (2005)
Article DOI: 10.1021/jm040178m
BindingDB Entry DOI: 10.7270/Q2HX1C66 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50167984

(CHEMBL370493 | K-252a analogue)Show SMILES CC1(C)O[C@H](C[C@@]1(O)CO)n1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C27H25N3O4/c1-26(2)27(33,13-31)11-19(34-26)30-18-10-6-4-8-15(18)21-22-16(12-28-25(22)32)20-14-7-3-5-9-17(14)29-23(20)24(21)30/h3-10,19,29,31,33H,11-13H2,1-2H3,(H,28,32)/t19-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of baculovirus expressed human VEGFR2 |
J Med Chem 48: 3776-83 (2005)
Article DOI: 10.1021/jm040178m
BindingDB Entry DOI: 10.7270/Q2HX1C66 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50118955

(CHEMBL94678 | Compound K-252a(epi))Show SMILES COC(=O)[C@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18-,26+,27-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NGF-stimulated TrkA phosphorylation in NIH3T3 cells |
J Med Chem 48: 3776-83 (2005)
Article DOI: 10.1021/jm040178m
BindingDB Entry DOI: 10.7270/Q2HX1C66 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 11
(Homo sapiens (Human)) | BDBM50108296

(CHEMBL289772 | Indolocarbazole analogue)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccc(CO)cc3c3c4CNC(=O)c4c4c5cc(CO)ccc5n2c4c13 Show InChI InChI=1S/C29H25N3O7/c1-28-29(37,27(36)38-2)9-20(39-28)31-18-5-3-13(11-33)7-15(18)22-23-17(10-30-26(23)35)21-16-8-14(12-34)4-6-19(16)32(28)25(21)24(22)31/h3-8,20,33-34,37H,9-12H2,1-2H3,(H,30,35)/t20?,28-,29-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 3 (MLK3) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 11
(Homo sapiens (Human)) | BDBM50108295
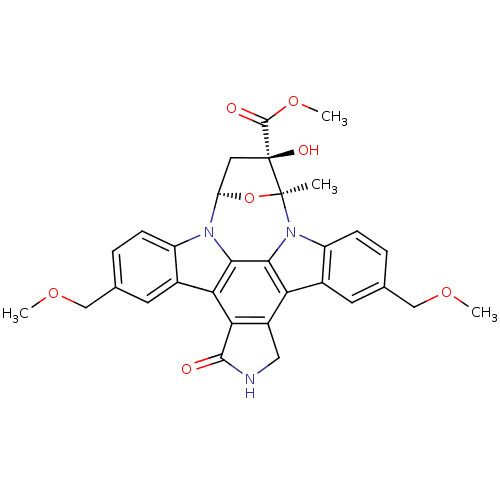
(CHEMBL288817 | Indolocarbazole analogue)Show SMILES COCc1ccc2n3[C@H]4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(COC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C31H29N3O7/c1-30-31(37,29(36)40-4)11-22(41-30)33-20-7-5-15(13-38-2)9-17(20)24-25-19(12-32-28(25)35)23-18-10-16(14-39-3)6-8-21(18)34(30)27(23)26(24)33/h5-10,22,37H,11-14H2,1-4H3,(H,32,35)/t22?,30-,31-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 3 (MLK3) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 11
(Homo sapiens (Human)) | BDBM50108293
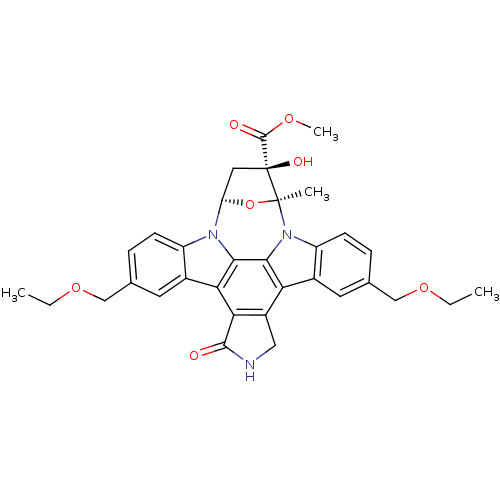
(CHEMBL277817 | Indolocarbazole analogue)Show SMILES CCOCc1ccc2n3[C@H]4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(COCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C33H33N3O7/c1-5-41-15-17-7-9-22-19(11-17)26-27-21(14-34-30(27)37)25-20-12-18(16-42-6-2)8-10-23(20)36-29(25)28(26)35(22)24-13-33(39,31(38)40-4)32(36,3)43-24/h7-12,24,39H,5-6,13-16H2,1-4H3,(H,34,37)/t24?,32-,33-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 3 (MLK3) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 11
(Homo sapiens (Human)) | BDBM14186
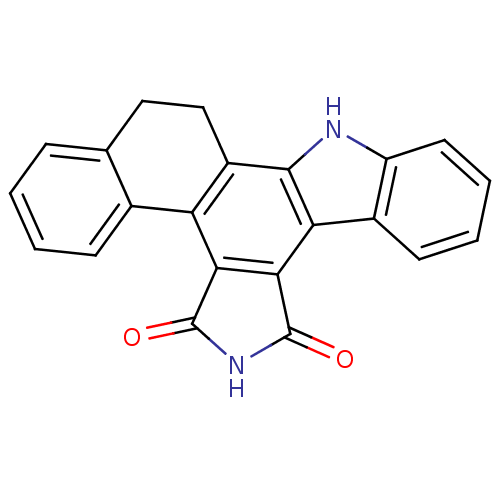
(12,13-Dihydro-6H,14H-naphthyl[3,4-a]pyrrolo[3,4-c]...)Show SMILES O=C1NC(=O)c2c1c-1c(CCc3ccccc-13)c1[nH]c3ccccc3c21 Show InChI InChI=1S/C22H14N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-8,23H,9-10H2,(H,24,25,26) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon
| Assay Description
The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate... |
J Med Chem 50: 433-41 (2007)
Article DOI: 10.1021/jm051074u
BindingDB Entry DOI: 10.7270/Q2HT2MJX |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of baculovirus expressed human trkA |
J Med Chem 48: 3776-83 (2005)
Article DOI: 10.1021/jm040178m
BindingDB Entry DOI: 10.7270/Q2HX1C66 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 11
(Homo sapiens (Human)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon
| Assay Description
The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate... |
J Med Chem 50: 433-41 (2007)
Article DOI: 10.1021/jm051074u
BindingDB Entry DOI: 10.7270/Q2HT2MJX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50108296

(CHEMBL289772 | Indolocarbazole analogue)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccc(CO)cc3c3c4CNC(=O)c4c4c5cc(CO)ccc5n2c4c13 Show InChI InChI=1S/C29H25N3O7/c1-28-29(37,27(36)38-2)9-20(39-28)31-18-5-3-13(11-33)7-15(18)22-23-17(10-30-26(23)35)21-16-8-14(12-34)4-6-19(16)32(28)25(21)24(22)31/h3-8,20,33-34,37H,9-12H2,1-2H3,(H,30,35)/t20?,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 1 (MLK1) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50108295

(CHEMBL288817 | Indolocarbazole analogue)Show SMILES COCc1ccc2n3[C@H]4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(COC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C31H29N3O7/c1-30-31(37,29(36)40-4)11-22(41-30)33-20-7-5-15(13-38-2)9-17(20)24-25-19(12-32-28(25)35)23-18-10-16(14-39-3)6-8-21(18)34(30)27(23)26(24)33/h5-10,22,37H,11-14H2,1-4H3,(H,32,35)/t22?,30-,31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 1 (MLK1) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 10
(Homo sapiens (Human)) | BDBM50108295

(CHEMBL288817 | Indolocarbazole analogue)Show SMILES COCc1ccc2n3[C@H]4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(COC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C31H29N3O7/c1-30-31(37,29(36)40-4)11-22(41-30)33-20-7-5-15(13-38-2)9-17(20)24-25-19(12-32-28(25)35)23-18-10-16(14-39-3)6-8-21(18)34(30)27(23)26(24)33/h5-10,22,37H,11-14H2,1-4H3,(H,32,35)/t22?,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 2(MLK2) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50108293

(CHEMBL277817 | Indolocarbazole analogue)Show SMILES CCOCc1ccc2n3[C@H]4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(COCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C33H33N3O7/c1-5-41-15-17-7-9-22-19(11-17)26-27-21(14-34-30(27)37)25-20-12-18(16-42-6-2)8-10-23(20)36-29(25)28(26)35(22)24-13-33(39,31(38)40-4)32(36,3)43-24/h7-12,24,39H,5-6,13-16H2,1-4H3,(H,34,37)/t24?,32-,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 1 (MLK1) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 10
(Homo sapiens (Human)) | BDBM50108293

(CHEMBL277817 | Indolocarbazole analogue)Show SMILES CCOCc1ccc2n3[C@H]4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(COCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 Show InChI InChI=1S/C33H33N3O7/c1-5-41-15-17-7-9-22-19(11-17)26-27-21(14-34-30(27)37)25-20-12-18(16-42-6-2)8-10-23(20)36-29(25)28(26)35(22)24-13-33(39,31(38)40-4)32(36,3)43-24/h7-12,24,39H,5-6,13-16H2,1-4H3,(H,34,37)/t24?,32-,33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 2(MLK2) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50410232

(CHEMBL2113062)Show SMILES C[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C26H21N3O3/c1-25(31)11-18-28-16-9-5-3-7-13(16)20-21-15(12-27-24(21)30)19-14-8-4-6-10-17(14)29(23(19)22(20)28)26(25,2)32-18/h3-10,18,31H,11-12H2,1-2H3,(H,27,30)/t18-,25-,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of baculovirus expressed human trkA |
J Med Chem 48: 3776-83 (2005)
Article DOI: 10.1021/jm040178m
BindingDB Entry DOI: 10.7270/Q2HX1C66 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 11
(Homo sapiens (Human)) | BDBM50108299

(CHEMBL288229 | Indolocarbazole analogue)Show SMILES CCSCc1ccc2n3c4c(c5CNC(=O)c5c5c6ccccc6n([C@H]6C[C@](O)(C(=O)OC)[C@]3(C)O6)c45)c2c1 Show InChI InChI=1S/C30H27N3O5S/c1-4-39-14-15-9-10-20-17(11-15)22-18-13-31-27(34)24(18)23-16-7-5-6-8-19(16)32-21-12-30(36,28(35)37-3)29(2,38-21)33(20)26(22)25(23)32/h5-11,21,36H,4,12-14H2,1-3H3,(H,31,34)/t21?,29-,30-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 3 (MLK3) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 11
(Homo sapiens (Human)) | BDBM14174
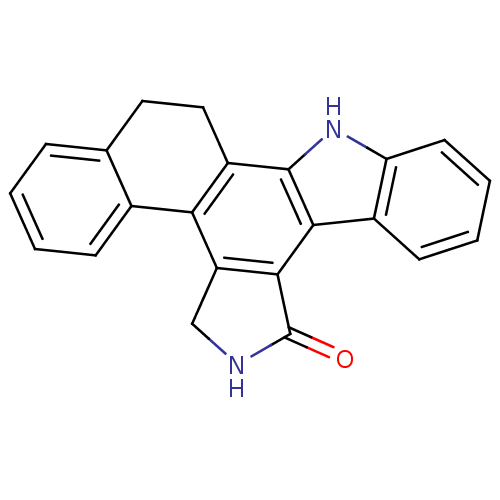
(12,13-Dihydro-5H,6H,14H-naphthy[3,4-a]pyrrolo[3,4-...)Show SMILES O=C1NCc2c1c1c([nH]c3ccccc13)c1CCc3ccccc3-c21 Show InChI InChI=1S/C22H16N2O/c25-22-20-16(11-23-22)18-13-6-2-1-5-12(13)9-10-15(18)21-19(20)14-7-3-4-8-17(14)24-21/h1-8,24H,9-11H2,(H,23,25) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon
| Assay Description
The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate... |
J Med Chem 50: 433-41 (2007)
Article DOI: 10.1021/jm051074u
BindingDB Entry DOI: 10.7270/Q2HT2MJX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM14186

(12,13-Dihydro-6H,14H-naphthyl[3,4-a]pyrrolo[3,4-c]...)Show SMILES O=C1NC(=O)c2c1c-1c(CCc3ccccc-13)c1[nH]c3ccccc3c21 Show InChI InChI=1S/C22H14N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-8,23H,9-10H2,(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Cephalon
| Assay Description
The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate... |
J Med Chem 50: 433-41 (2007)
Article DOI: 10.1021/jm051074u
BindingDB Entry DOI: 10.7270/Q2HT2MJX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM6760

((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...)Show SMILES COC(=O)[C@@]1(O)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Cephalon
| Assay Description
The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate... |
J Med Chem 50: 433-41 (2007)
Article DOI: 10.1021/jm051074u
BindingDB Entry DOI: 10.7270/Q2HT2MJX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 11
(Homo sapiens (Human)) | BDBM24942
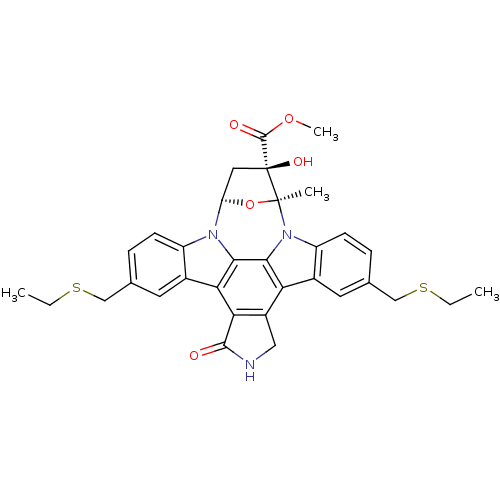
(CEP-1347 | CHEMBL290352 | methyl (15S,16R,18R)-10,...)Show SMILES CCSCc1ccc2n3[C@H]4C[C@](O)(C(=O)OC)[C@](C)(O4)n4c5ccc(CSCC)cc5c5c6CNC(=O)c6c(c2c1)c3c45 |r| Show InChI InChI=1S/C33H33N3O5S2/c1-5-42-15-17-7-9-22-19(11-17)26-27-21(14-34-30(27)37)25-20-12-18(16-43-6-2)8-10-23(20)36-29(25)28(26)35(22)24-13-33(39,31(38)40-4)32(36,3)41-24/h7-12,24,39H,5-6,13-16H2,1-4H3,(H,34,37)/t24-,32+,33+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 3 (MLK3) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 11
(Homo sapiens (Human)) | BDBM14179
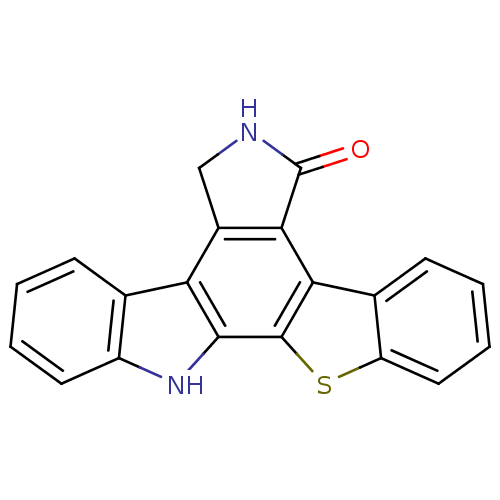
(3-thia-13,23-diazahexacyclo[14.7.0.0^{2,10}.0^{4,9...)Show InChI InChI=1S/C20H12N2OS/c23-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)24-19/h1-8,22H,9H2,(H,21,23) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon
| Assay Description
The MLK1, MLK2, and MLK3 assays were performed using the Millipore multiscreen trichloroacetic acid (TCA) in-plate format. IC50 values were calculate... |
J Med Chem 50: 433-41 (2007)
Article DOI: 10.1021/jm051074u
BindingDB Entry DOI: 10.7270/Q2HT2MJX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 10
(Homo sapiens (Human)) | BDBM50108299

(CHEMBL288229 | Indolocarbazole analogue)Show SMILES CCSCc1ccc2n3c4c(c5CNC(=O)c5c5c6ccccc6n([C@H]6C[C@](O)(C(=O)OC)[C@]3(C)O6)c45)c2c1 Show InChI InChI=1S/C30H27N3O5S/c1-4-39-14-15-9-10-20-17(11-15)22-18-13-31-27(34)24(18)23-16-7-5-6-8-19(16)32-21-12-30(36,28(35)37-3)29(2,38-21)33(20)26(22)25(23)32/h5-11,21,36H,4,12-14H2,1-3H3,(H,31,34)/t21?,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 2(MLK2) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50108299

(CHEMBL288229 | Indolocarbazole analogue)Show SMILES CCSCc1ccc2n3c4c(c5CNC(=O)c5c5c6ccccc6n([C@H]6C[C@](O)(C(=O)OC)[C@]3(C)O6)c45)c2c1 Show InChI InChI=1S/C30H27N3O5S/c1-4-39-14-15-9-10-20-17(11-15)22-18-13-31-27(34)24(18)23-16-7-5-6-8-19(16)32-21-12-30(36,28(35)37-3)29(2,38-21)33(20)26(22)25(23)32/h5-11,21,36H,4,12-14H2,1-3H3,(H,31,34)/t21?,29-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa-Hakko Kogyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Mixed lineage kinase 1 (MLK1) |
Bioorg Med Chem Lett 12: 147-50 (2001)
BindingDB Entry DOI: 10.7270/Q2571B94 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50410234

(CHEMBL2113065)Show SMILES C[C@]12O[C@H](C[C@@]1(O)C(N)=O)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C26H20N4O4/c1-25-26(33,24(27)32)10-17(34-25)29-15-8-4-2-6-12(15)19-20-14(11-28-23(20)31)18-13-7-3-5-9-16(13)30(25)22(18)21(19)29/h2-9,17,33H,10-11H2,1H3,(H2,27,32)(H,28,31)/t17-,25+,26-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Cephalon, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of baculovirus expressed human trkA |
J Med Chem 48: 3776-83 (2005)
Article DOI: 10.1021/jm040178m
BindingDB Entry DOI: 10.7270/Q2HX1C66 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data