| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50558880 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2066468 (CHEMBL4721721) |
|---|
| IC50 | >25000±n/a nM |
|---|
| Citation |  Curreli, F; Belov, DS; Kwon, YD; Ramesh, R; Furimsky, AM; O'Loughlin, K; Byrge, PC; Iyer, LV; Mirsalis, JC; Kurkin, AV; Altieri, A; Debnath, AK Structure-based lead optimization to improve antiviral potency and ADMET properties of phenyl-1H-pyrrole-carboxamide entry inhibitors targeted to HIV-1 gp120. Eur J Med Chem154:367-391 (2018) [PubMed] Article Curreli, F; Belov, DS; Kwon, YD; Ramesh, R; Furimsky, AM; O'Loughlin, K; Byrge, PC; Iyer, LV; Mirsalis, JC; Kurkin, AV; Altieri, A; Debnath, AK Structure-based lead optimization to improve antiviral potency and ADMET properties of phenyl-1H-pyrrole-carboxamide entry inhibitors targeted to HIV-1 gp120. Eur J Med Chem154:367-391 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50558880 |
|---|
| n/a |
|---|
| Name | BDBM50558880 |
|---|
| Synonyms: | CHEMBL4533529 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H16F4N4O2S |
|---|
| Mol. Mass. | 428.404 |
|---|
| SMILES | NC[C@H](NC(=O)c1ccc([nH]1)-c1ccc(c(F)c1)C(F)(F)F)c1nc(CO)cs1 |r| |
|---|
| Structure | 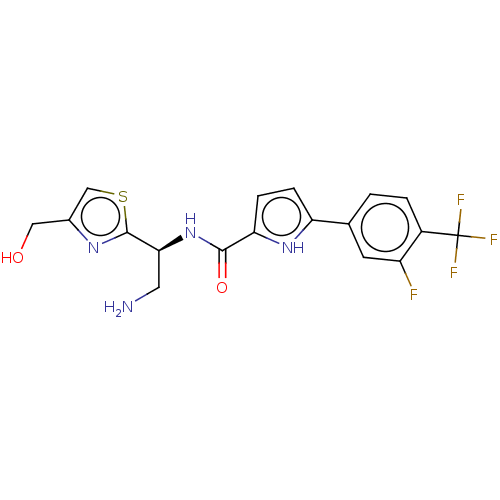
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Curreli, F; Belov, DS; Kwon, YD; Ramesh, R; Furimsky, AM; O'Loughlin, K; Byrge, PC; Iyer, LV; Mirsalis, JC; Kurkin, AV; Altieri, A; Debnath, AK Structure-based lead optimization to improve antiviral potency and ADMET properties of phenyl-1H-pyrrole-carboxamide entry inhibitors targeted to HIV-1 gp120. Eur J Med Chem154:367-391 (2018) [PubMed] Article
Curreli, F; Belov, DS; Kwon, YD; Ramesh, R; Furimsky, AM; O'Loughlin, K; Byrge, PC; Iyer, LV; Mirsalis, JC; Kurkin, AV; Altieri, A; Debnath, AK Structure-based lead optimization to improve antiviral potency and ADMET properties of phenyl-1H-pyrrole-carboxamide entry inhibitors targeted to HIV-1 gp120. Eur J Med Chem154:367-391 (2018) [PubMed] Article