

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288943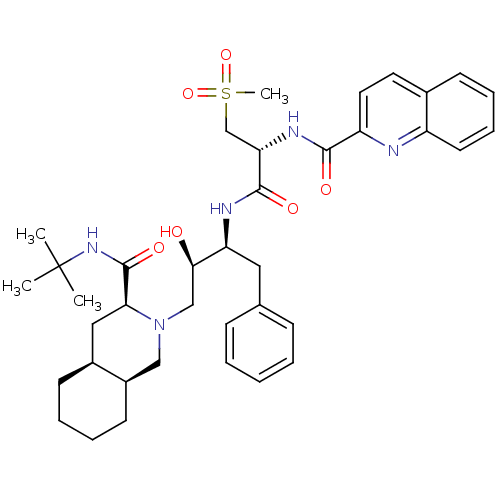 (CHEMBL154519 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50454862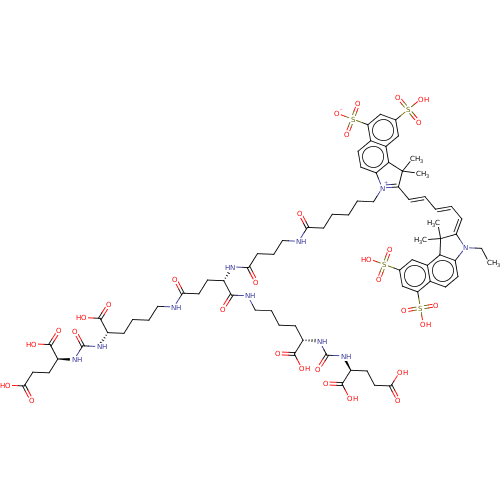 (CHEMBL4211875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell lysates incubated for 2 hrs in presence of N-acetylaspartylglutamate by Amplex red glutamic acid/glutamate oxi... | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288941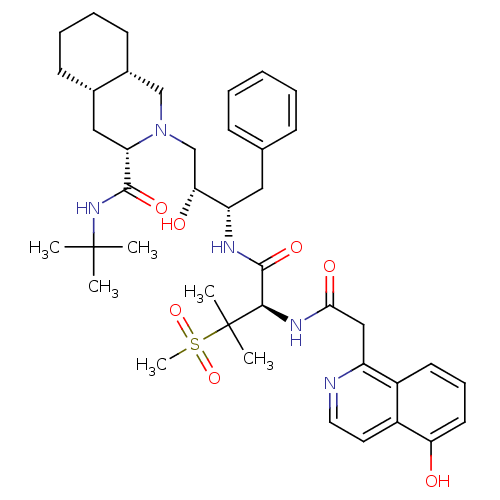 ((3S,4aS,8aS)-2-((2R,3S)-2-Hydroxy-3-{(R)-2-[2-(5-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288942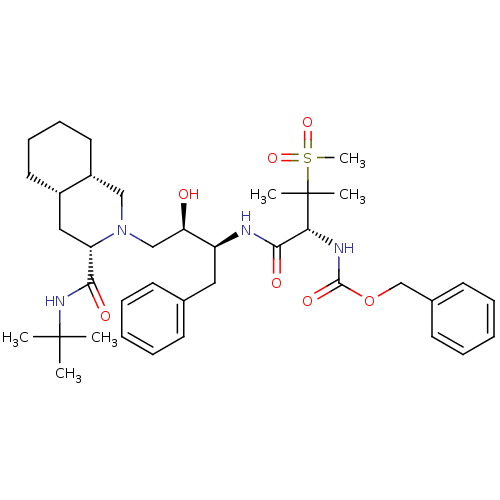 (CHEMBL154692 | {(R)-1-[(1S,2R)-1-Benzyl-3-((3S,4aS...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288939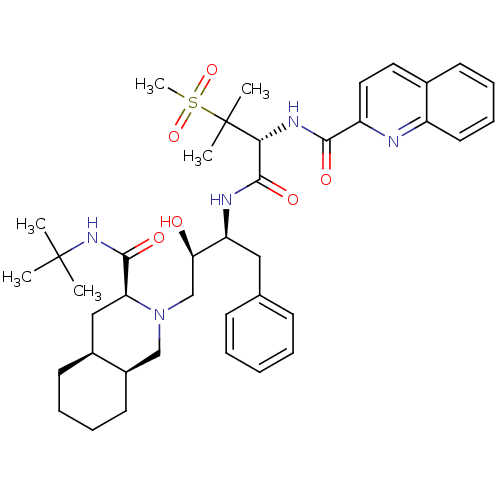 (CHEMBL345187 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50454861 (CHEMBL4206989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell lysates incubated for 2 hrs in presence of N-acetylaspartylglutamate by Amplex red glutamic acid/glutamate oxi... | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9294 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50454860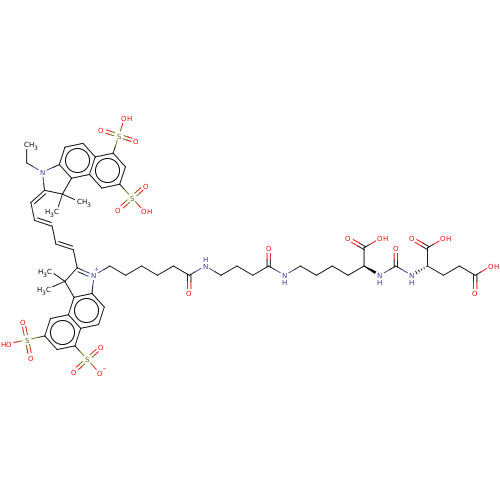 (CHEMBL4202635) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine Curated by ChEMBL | Assay Description Inhibition of PSMA in human LNCaP cell lysates incubated for 2 hrs in presence of N-acetylaspartylglutamate by Amplex red glutamic acid/glutamate oxi... | Bioorg Med Chem Lett 28: 572-576 (2018) Article DOI: 10.1016/j.bmcl.2018.01.047 BindingDB Entry DOI: 10.7270/Q2RN3BGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288940 (CHEMBL154416 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50558881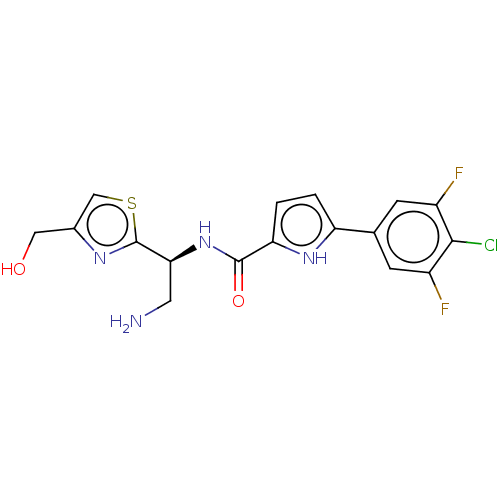 (CHEMBL4761836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG transfected in HEK293 cells assessed as reduction in channel current at -80 mV holding potential by whole-cell patch clamp me... | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50558880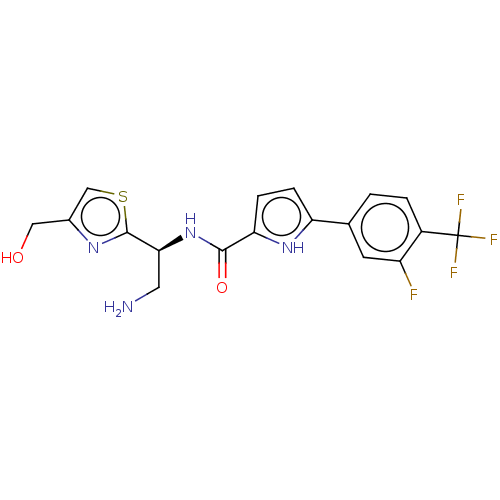 (CHEMBL4533529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG transfected in HEK293 cells assessed as reduction in channel current at -80 mV holding potential by whole-cell patch clamp me... | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Envelope glycoprotein gp160 (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50333307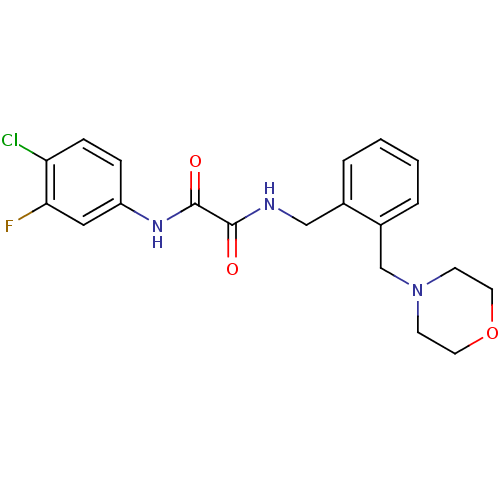 (CHEMBL1645291 | N1-(4-chloro-3-fluorophenyl)-N2-(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of HIV1 YU2 gp120 binding to CD4 expressing Cf2Th-CD4/CCR5 cells assessed as inhibition of viral infection after 48 hrs | Bioorg Med Chem 19: 91-101 (2011) Article DOI: 10.1016/j.bmc.2010.11.049 BindingDB Entry DOI: 10.7270/Q25H7GHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50236759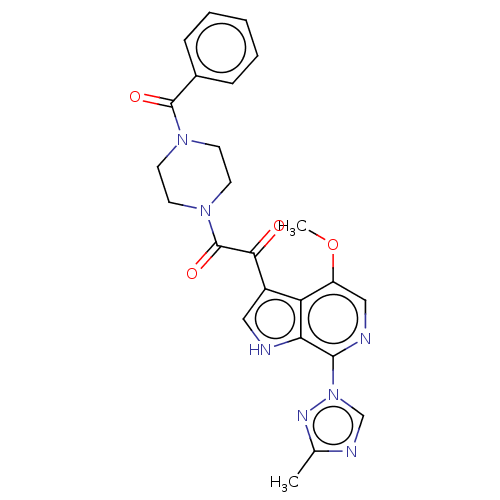 (BMS-626529 | Temsavir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG transfected in HEK293 cells assessed as reduction in channel current at -80 mV holding potential by whole-cell patch clamp me... | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50237125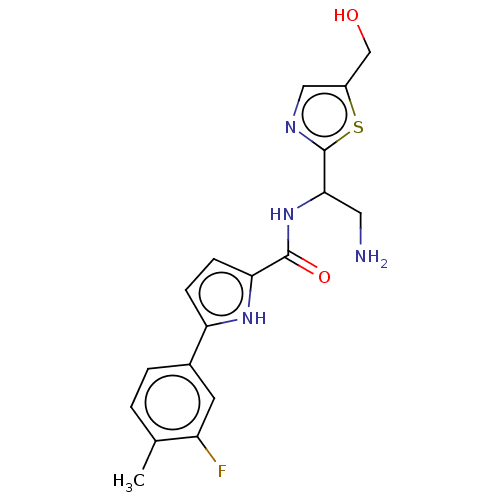 (CHEMBL4075766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 5 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50558881 (CHEMBL4761836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP3A4 using midazolam as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50558880 (CHEMBL4533529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP3A4 using midazolam as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50236759 (BMS-626529 | Temsavir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP3A4 using midazolam as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50558881 (CHEMBL4761836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP3A4 using testosterone as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50558880 (CHEMBL4533529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP3A4 using testosterone as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50236759 (BMS-626529 | Temsavir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP3A4 using testosterone as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50558881 (CHEMBL4761836) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2D6 using bufuralol as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50558880 (CHEMBL4533529) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2D6 using bufuralol as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50236759 (BMS-626529 | Temsavir) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2D6 using bufuralol as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50558881 (CHEMBL4761836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C19 using mephenytoin as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50558880 (CHEMBL4533529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C19 using mephenytoin as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50236759 (BMS-626529 | Temsavir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C19 using mephenytoin as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50558881 (CHEMBL4761836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C9 using diclofenac as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50558880 (CHEMBL4533529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C9 using diclofenac as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50236759 (BMS-626529 | Temsavir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C9 using diclofenac as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50558881 (CHEMBL4761836) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C8 using paclitaxel as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50558880 (CHEMBL4533529) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C8 using paclitaxel as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50236759 (BMS-626529 | Temsavir) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2C8 using paclitaxel as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50558881 (CHEMBL4761836) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human liver microsome CYP2B6 using bupropion as substrate incubated for 20 mins by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2018.04.062 BindingDB Entry DOI: 10.7270/Q2183B73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50237224 (CHEMBL4083672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate after 60 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50236759 (BMS-626529 | Temsavir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 5 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50236717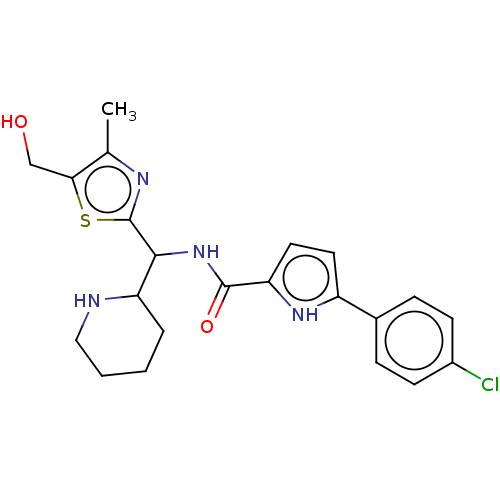 (CHEMBL4096918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate after 60 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50236759 (BMS-626529 | Temsavir) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 5 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50236759 (BMS-626529 | Temsavir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate after 60 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50237224 (CHEMBL4083672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 5 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50236759 (BMS-626529 | Temsavir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 60 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50237125 (CHEMBL4075766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 60 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50236717 (CHEMBL4096918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 5 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50237125 (CHEMBL4075766) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 5 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50237125 (CHEMBL4075766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate after 60 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50236717 (CHEMBL4096918) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 5 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50237224 (CHEMBL4083672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 60 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50237224 (CHEMBL4083672) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 5 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50236717 (CHEMBL4096918) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsey F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 60 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 60: 3124-3153 (2017) Article DOI: 10.1021/acs.jmedchem.7b00179 BindingDB Entry DOI: 10.7270/Q2XD13Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 220 total ) | Next | Last >> |