| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Muscarinic acetylcholine receptor M2 |
|---|
| Ligand | BDBM50123443 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_138182 |
|---|
| Ki | 169±n/a nM |
|---|
| Citation |  McCombie, SW; Tagat, JR; Vice, SF; Lin, SI; Steensma, R; Palani, A; Neustadt, BR; Baroudy, BM; Strizki, JM; Endres, M; Cox, K; Dan, N; Chou, CC Piperazine-based CCR5 antagonists as HIV-1 inhibitors. III: synthesis, antiviral and pharmacokinetic profiles of symmetrical heteroaryl carboxamides. Bioorg Med Chem Lett13:567-71 (2003) [PubMed] McCombie, SW; Tagat, JR; Vice, SF; Lin, SI; Steensma, R; Palani, A; Neustadt, BR; Baroudy, BM; Strizki, JM; Endres, M; Cox, K; Dan, N; Chou, CC Piperazine-based CCR5 antagonists as HIV-1 inhibitors. III: synthesis, antiviral and pharmacokinetic profiles of symmetrical heteroaryl carboxamides. Bioorg Med Chem Lett13:567-71 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Muscarinic acetylcholine receptor M2 |
|---|
| Name: | Muscarinic acetylcholine receptor M2 |
|---|
| Synonyms: | ACM2_HUMAN | CHRM2 | Cholinergic, muscarinic M2 | Muscarinic acetylcholine receptor M2 and M4 | Muscarinic acetylcholine receptor M2 and M5 | RecName: Full=Muscarinic acetylcholine receptor M2 |
|---|
| Type: | GPCR |
|---|
| Mol. Mass.: | 51730.61 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P08172 |
|---|
| Residue: | 466 |
|---|
| Sequence: | MNNSTNSSNNSLALTSPYKTFEVVFIVLVAGSLSLVTIIGNILVMVSIKVNRHLQTVNNY
FLFSLACADLIIGVFSMNLYTLYTVIGYWPLGPVVCDLWLALDYVVSNASVMNLLIISFD
RYFCVTKPLTYPVKRTTKMAGMMIAAAWVLSFILWAPAILFWQFIVGVRTVEDGECYIQF
FSNAAVTFGTAIAAFYLPVIIMTVLYWHISRASKSRIKKDKKEPVANQDPVSPSLVQGRI
VKPNNNNMPSSDDGLEHNKIQNGKAPRDPVTENCVQGEEKESSNDSTSVSAVASNMRDDE
ITQDENTVSTSLGHSKDENSKQTCIRIGTKTPKSDSCTPTNTTVEVVGSSGQNGDEKQNI
VARKIVKMTKQPAKKKPPPSREKKVTRTILAILLAFIITWAPYNVMVLINTFCAPCIPNT
VWTIGYWLCYINSTINPACYALCNATFKKTFKHLLMCHYKNIGATR
|
|
|
|---|
| BDBM50123443 |
|---|
| n/a |
|---|
| Name | BDBM50123443 |
|---|
| Synonyms: | (4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(S)-1-(4-iodo-phenyl)-ethyl]-3-methyl-piperazin-1-yl}-4-methyl-piperidin-1-yl)-methanone | CHEMBL422326 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H36IN5O |
|---|
| Mol. Mass. | 561.5014 |
|---|
| SMILES | C[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(I)cc1 |
|---|
| Structure | 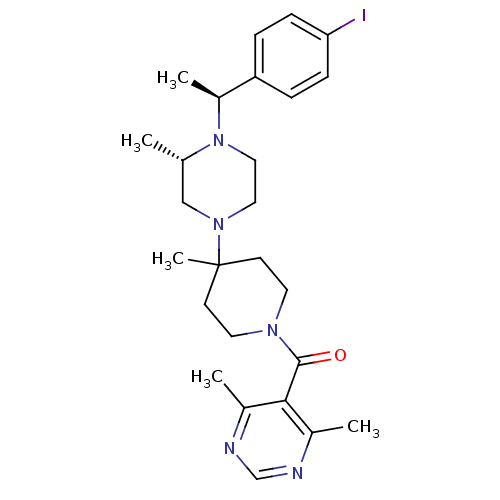
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 McCombie, SW; Tagat, JR; Vice, SF; Lin, SI; Steensma, R; Palani, A; Neustadt, BR; Baroudy, BM; Strizki, JM; Endres, M; Cox, K; Dan, N; Chou, CC Piperazine-based CCR5 antagonists as HIV-1 inhibitors. III: synthesis, antiviral and pharmacokinetic profiles of symmetrical heteroaryl carboxamides. Bioorg Med Chem Lett13:567-71 (2003) [PubMed]
McCombie, SW; Tagat, JR; Vice, SF; Lin, SI; Steensma, R; Palani, A; Neustadt, BR; Baroudy, BM; Strizki, JM; Endres, M; Cox, K; Dan, N; Chou, CC Piperazine-based CCR5 antagonists as HIV-1 inhibitors. III: synthesis, antiviral and pharmacokinetic profiles of symmetrical heteroaryl carboxamides. Bioorg Med Chem Lett13:567-71 (2003) [PubMed]