

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123438 ((3,5-Dichloro-pyridin-4-yl)-(4-methyl-4-{(S)-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104946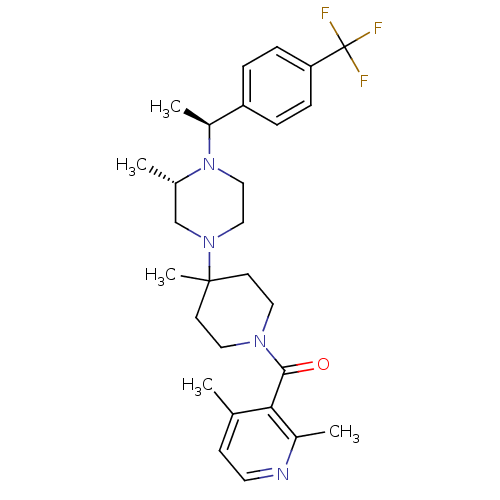 ((2,4-Dimethyl-pyridin-3-yl)-(4-methyl-4-{(S)-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123436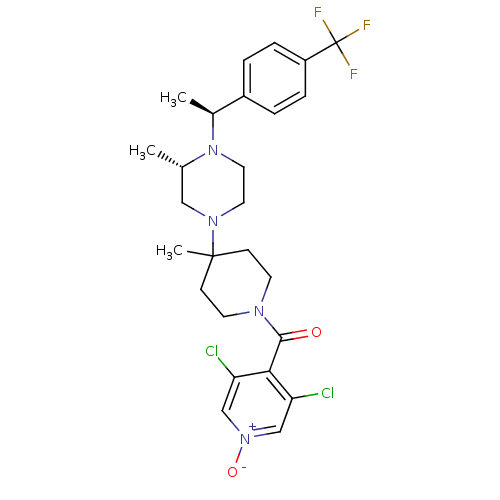 ((3,5-Dichloro-1-oxy-pyridin-4-yl)-(4-methyl-4-{(S)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123435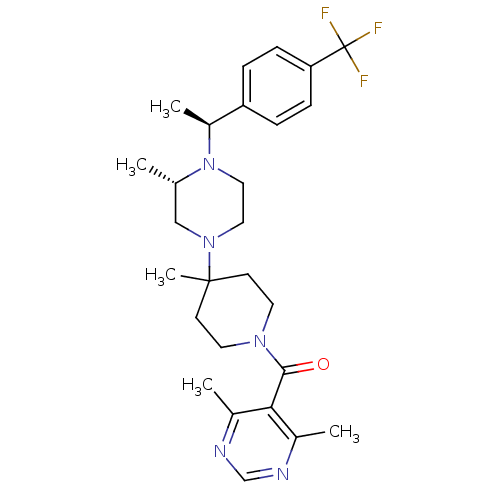 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability of compound to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123445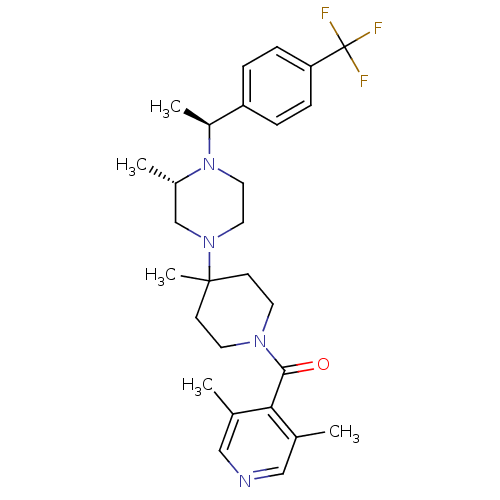 ((3,5-Dimethyl-pyridin-4-yl)-(4-methyl-4-{(S)-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104956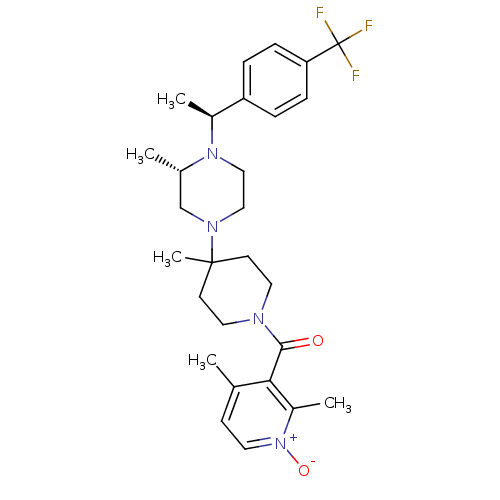 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-methyl-4-{(S)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123444 ((4,6-Dimethyl-2-trifluoromethyl-pyrimidin-5-yl)-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123442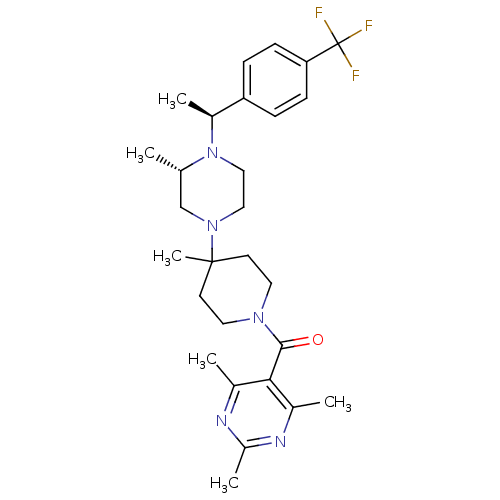 ((4-Methyl-4-{(S)-3-methyl-4-[(S)-1-(4-trifluoromet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123443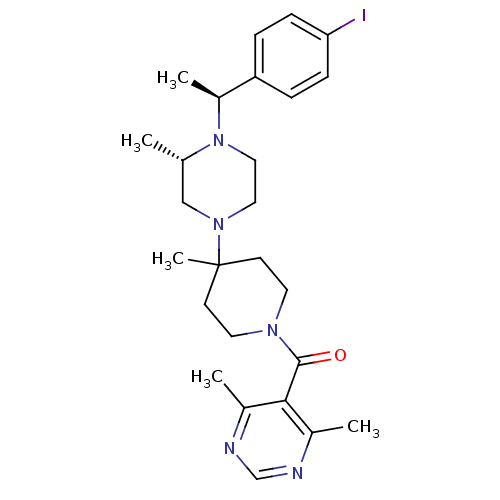 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(S)-1-(4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123437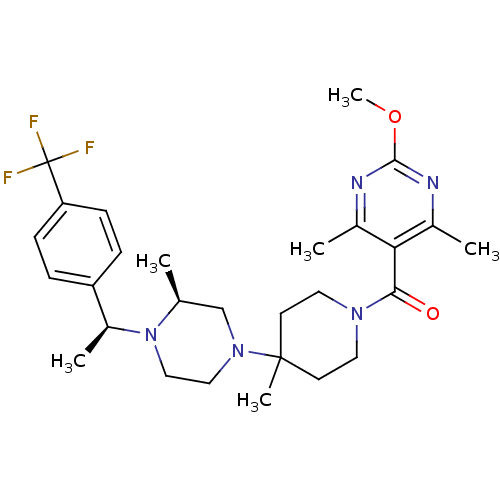 ((2-Methoxy-4,6-dimethyl-pyrimidin-5-yl)-(4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123446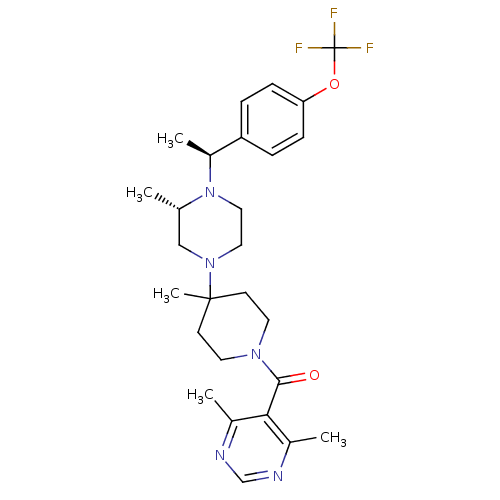 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123439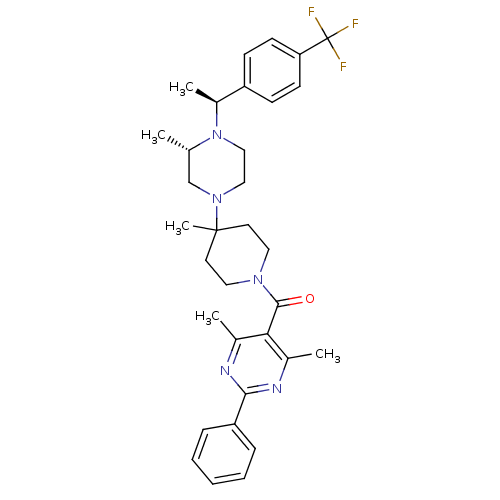 ((4,6-Dimethyl-2-phenyl-pyrimidin-5-yl)-(4-methyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123447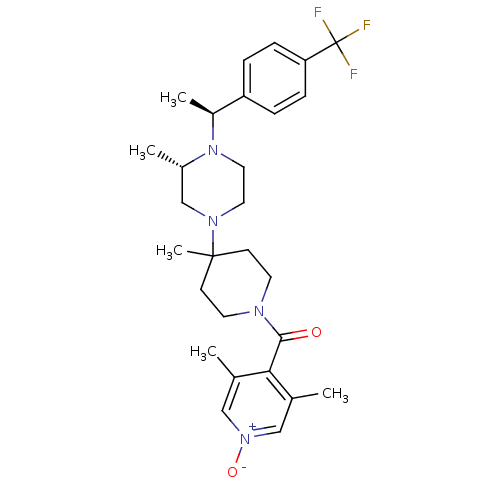 ((3,5-Dimethyl-1-oxy-pyridin-4-yl)-(4-methyl-4-{(S)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123440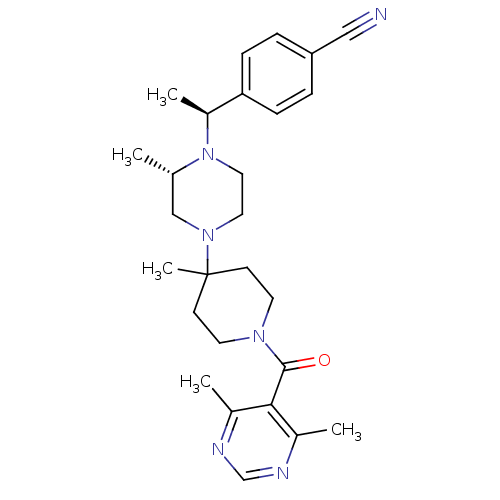 (4-((S)-1-{(S)-4-[1-(4,6-Dimethyl-pyrimidine-5-carb...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123441 ((2-Amino-4,6-dimethyl-pyrimidin-5-yl)-(4-methyl-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123446 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123440 (4-((S)-1-{(S)-4-[1-(4,6-Dimethyl-pyrimidine-5-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123443 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(S)-1-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123437 ((2-Methoxy-4,6-dimethyl-pyrimidin-5-yl)-(4-methyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50104956 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-methyl-4-{(S)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123447 ((3,5-Dimethyl-1-oxy-pyridin-4-yl)-(4-methyl-4-{(S)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123436 ((3,5-Dichloro-1-oxy-pyridin-4-yl)-(4-methyl-4-{(S)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 348 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50104956 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-methyl-4-{(S)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards muscarinic receptor M1 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of binding affinity of the compound to muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123444 ((4,6-Dimethyl-2-trifluoromethyl-pyrimidin-5-yl)-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 613 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123442 ((4-Methyl-4-{(S)-3-methyl-4-[(S)-1-(4-trifluoromet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 985 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123441 ((2-Amino-4,6-dimethyl-pyrimidin-5-yl)-(4-methyl-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123439 ((4,6-Dimethyl-2-phenyl-pyrimidin-5-yl)-(4-methyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123445 ((3,5-Dimethyl-pyridin-4-yl)-(4-methyl-4-{(S)-3-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123438 ((3,5-Dichloro-pyridin-4-yl)-(4-methyl-4-{(S)-3-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50104946 ((2,4-Dimethyl-pyridin-3-yl)-(4-methyl-4-{(S)-3-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards muscarinic receptor M2 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of 0.1 nM of MIP-1beta induced migration of recombinant mouse pro-B cell line BA/F3 expressing human CCR5 | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin-linked protein kinase (Homo sapiens (Human)) | BDBM50353484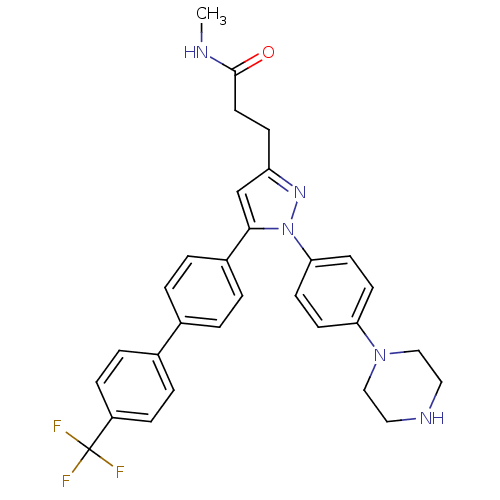 (CHEMBL1830587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of human ILK activity assessed myelin basic protein phosphorylation by radiometric kinase assay in the presence of [gamma 32P]ATP | J Med Chem 54: 6364-74 (2011) Article DOI: 10.1021/jm2007744 BindingDB Entry DOI: 10.7270/Q2513ZKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1beta ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1beta ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153764 ((Z)-1-((1S,2S)-2-((S)-hydroxy((R)-6-oxo-3,6-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1beta ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of 50% of RANTES co-receptor of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1alpha ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES co-receptor of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153764 ((Z)-1-((1S,2S)-2-((S)-hydroxy((R)-6-oxo-3,6-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1alpha ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153764 ((Z)-1-((1S,2S)-2-((S)-hydroxy((R)-6-oxo-3,6-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES co-receptor of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1alpha ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50078218 (CHEMBL3414677) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity to GST-tagged PH domain of AKT (unknown origin) by surface Plasmon resonance spectroscopy | J Med Chem 58: 2290-8 (2015) Article DOI: 10.1021/jm501751b BindingDB Entry DOI: 10.7270/Q20V8FGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50078219 (CHEMBL3414676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity to GST-tagged PH domain of AKT (unknown origin) by surface Plasmon resonance spectroscopy | J Med Chem 58: 2290-8 (2015) Article DOI: 10.1021/jm501751b BindingDB Entry DOI: 10.7270/Q20V8FGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50078220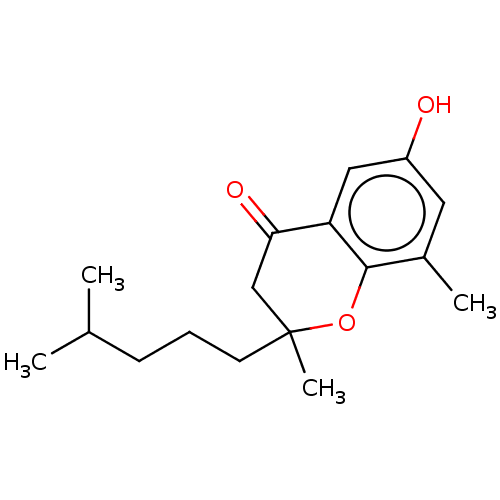 (CHEMBL3414675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity to GST-tagged PH domain of AKT (unknown origin) by surface Plasmon resonance spectroscopy | J Med Chem 58: 2290-8 (2015) Article DOI: 10.1021/jm501751b BindingDB Entry DOI: 10.7270/Q20V8FGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50078221 (CHEMBL3414674) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity to GST-tagged PH domain of AKT (unknown origin) by surface Plasmon resonance spectroscopy | J Med Chem 58: 2290-8 (2015) Article DOI: 10.1021/jm501751b BindingDB Entry DOI: 10.7270/Q20V8FGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50078222 (CHEMBL3414673) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity to GST-tagged PH domain of AKT (unknown origin) by surface Plasmon resonance spectroscopy | J Med Chem 58: 2290-8 (2015) Article DOI: 10.1021/jm501751b BindingDB Entry DOI: 10.7270/Q20V8FGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50078223 (CHEMBL3414672) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity to GST-tagged PH domain of AKT (unknown origin) by surface Plasmon resonance spectroscopy | J Med Chem 58: 2290-8 (2015) Article DOI: 10.1021/jm501751b BindingDB Entry DOI: 10.7270/Q20V8FGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50078256 (CHEMBL3414671) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity to GST-tagged PH domain of AKT (unknown origin) by surface Plasmon resonance spectroscopy | J Med Chem 58: 2290-8 (2015) Article DOI: 10.1021/jm501751b BindingDB Entry DOI: 10.7270/Q20V8FGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 72 total ) | Next | Last >> |