| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Endothelin receptor type B |
|---|
| Ligand | BDBM50124456 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_63688 |
|---|
| IC50 | 920±n/a nM |
|---|
| Citation |  Bolli, MH; Boss, C; Clozel, M; Fischli, W; Hess, P; Weller, T The use of sulfonylamido pyrimidines incorporating an unsaturated side chain as endothelin receptor antagonists. Bioorg Med Chem Lett13:955-9 (2003) [PubMed] Bolli, MH; Boss, C; Clozel, M; Fischli, W; Hess, P; Weller, T The use of sulfonylamido pyrimidines incorporating an unsaturated side chain as endothelin receptor antagonists. Bioorg Med Chem Lett13:955-9 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Endothelin receptor type B |
|---|
| Name: | Endothelin receptor type B |
|---|
| Synonyms: | EDNRB | EDNRB_HUMAN | ENDOTHELIN B | ET-B | ETRB | Endothelin receptor ET-B | Endothelin receptor non-selective type | Endothelin receptor, ET-A/ET-B |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49664.00 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ENDOTHELIN B EDNRB HUMAN::P24530 |
|---|
| Residue: | 442 |
|---|
| Sequence: | MQPPPSLCGRALVALVLACGLSRIWGEERGFPPDRATPLLQTAEIMTPPTKTLWPKGSNA
SLARSLAPAEVPKGDRTAGSPPRTISPPPCQGPIEIKETFKYINTVVSCLVFVLGIIGNS
TLLRIIYKNKCMRNGPNILIASLALGDLLHIVIDIPINVYKLLAEDWPFGAEMCKLVPFI
QKASVGITVLSLCALSIDRYRAVASWSRIKGIGVPKWTAVEIVLIWVVSVVLAVPEAIGF
DIITMDYKGSYLRICLLHPVQKTAFMQFYKTAKDWWLFSFYFCLPLAITAFFYTLMTCEM
LRKKSGMQIALNDHLKQRREVAKTVFCLVLVFALCWLPLHLSRILKLTLYNQNDPNRCEL
LSFLLVLDYIGINMASLNSCINPIALYLVSKRFKNCFKSCLCCWCQSFEEKQSLEEKQSC
LKFKANDHGYDNFRSSNKYSSS
|
|
|
|---|
| BDBM50124456 |
|---|
| n/a |
|---|
| Name | BDBM50124456 |
|---|
| Synonyms: | CHEMBL176741 | Pyridin-3-yl-carbamic acid 4-[5-(2-methoxy-phenoxy)-6-(5-methyl-pyridine-2-sulfonylamino)-2-morpholin-4-yl-pyrimidin-4-yloxy]-but-2-ynyl ester |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H31N7O8S |
|---|
| Mol. Mass. | 661.685 |
|---|
| SMILES | COc1ccccc1Oc1c(NS(=O)(=O)c2ccc(C)cn2)nc(nc1OCC#CCOC(=O)Nc1cccnc1)N1CCOCC1 |
|---|
| Structure | 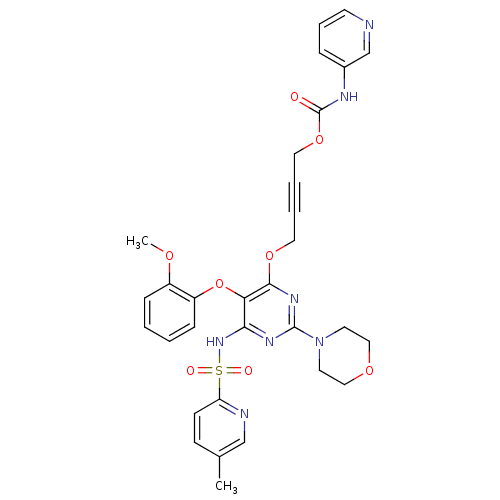
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bolli, MH; Boss, C; Clozel, M; Fischli, W; Hess, P; Weller, T The use of sulfonylamido pyrimidines incorporating an unsaturated side chain as endothelin receptor antagonists. Bioorg Med Chem Lett13:955-9 (2003) [PubMed]
Bolli, MH; Boss, C; Clozel, M; Fischli, W; Hess, P; Weller, T The use of sulfonylamido pyrimidines incorporating an unsaturated side chain as endothelin receptor antagonists. Bioorg Med Chem Lett13:955-9 (2003) [PubMed]