 Found 1900 hits with Last Name = 'hess' and Initial = 'p'
Found 1900 hits with Last Name = 'hess' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160917
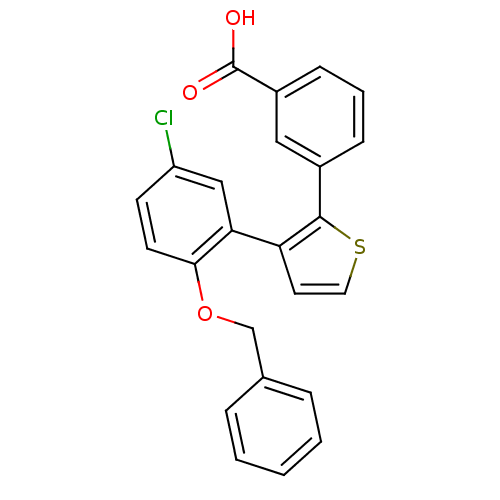
(3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...)Show SMILES OC(=O)c1cccc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C24H17ClO3S/c25-19-9-10-22(28-15-16-5-2-1-3-6-16)21(14-19)20-11-12-29-23(20)17-7-4-8-18(13-17)24(26)27/h1-14H,15H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against PGE2 activated EP1 receptor assessed as ability to inhibit intracellular calcium mobilisation by FLIPR |
Bioorg Med Chem Lett 16: 2666-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.014
BindingDB Entry DOI: 10.7270/Q2J102RM |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM86516

(ETPDCFWKYCV | Human U-II | L-Ala-Gly-L-Thr-L-Ala-L...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CS)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O)C(O)=O Show InChI InChI=1S/C62H86N14O17S2/c1-31(2)50(62(92)93)76-60(90)47(30-95)74-56(86)43(24-36-18-20-38(78)21-19-36)70-54(84)41(17-11-12-22-63)68-57(87)44(25-37-27-65-40-16-10-9-15-39(37)40)72-55(85)42(23-35-13-7-6-8-14-35)71-59(89)46(29-94)73-58(88)45(26-49(80)81)69-53(83)33(4)67-61(91)51(34(5)77)75-48(79)28-66-52(82)32(3)64/h6-10,13-16,18-21,27,31-34,41-47,50-51,65,77-78,94-95H,11-12,17,22-26,28-30,63-64H2,1-5H3,(H,66,82)(H,67,91)(H,68,87)(H,69,83)(H,70,84)(H,71,89)(H,72,85)(H,73,88)(H,74,86)(H,75,79)(H,76,90)(H,80,81)(H,92,93)/t32-,33-,34+,41-,42-,43-,44-,45-,46-,47-,50-,51-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 311: 204-12 (2004)
Article DOI: 10.1124/jpet.104.068320
BindingDB Entry DOI: 10.7270/Q2H993RW |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM86516

(ETPDCFWKYCV | Human U-II | L-Ala-Gly-L-Thr-L-Ala-L...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CS)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O)C(O)=O Show InChI InChI=1S/C62H86N14O17S2/c1-31(2)50(62(92)93)76-60(90)47(30-95)74-56(86)43(24-36-18-20-38(78)21-19-36)70-54(84)41(17-11-12-22-63)68-57(87)44(25-37-27-65-40-16-10-9-15-39(37)40)72-55(85)42(23-35-13-7-6-8-14-35)71-59(89)46(29-94)73-58(88)45(26-49(80)81)69-53(83)33(4)67-61(91)51(34(5)77)75-48(79)28-66-52(82)32(3)64/h6-10,13-16,18-21,27,31-34,41-47,50-51,65,77-78,94-95H,11-12,17,22-26,28-30,63-64H2,1-5H3,(H,66,82)(H,67,91)(H,68,87)(H,69,83)(H,70,84)(H,71,89)(H,72,85)(H,73,88)(H,74,86)(H,75,79)(H,76,90)(H,80,81)(H,92,93)/t32-,33-,34+,41-,42-,43-,44-,45-,46-,47-,50-,51-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 311: 204-12 (2004)
Article DOI: 10.1124/jpet.104.068320
BindingDB Entry DOI: 10.7270/Q2H993RW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461458
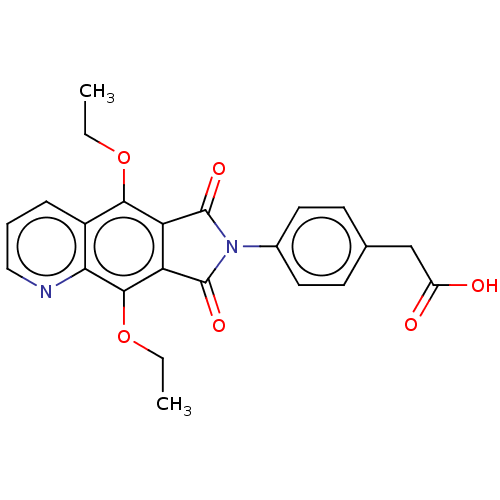
(CHEMBL4229200)Show SMILES CCOc1c2C(=O)N(C(=O)c2c(OCC)c2ncccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C23H20N2O6/c1-3-30-20-15-6-5-11-24-19(15)21(31-4-2)18-17(20)22(28)25(23(18)29)14-9-7-13(8-10-14)12-16(26)27/h5-11H,3-4,12H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461462
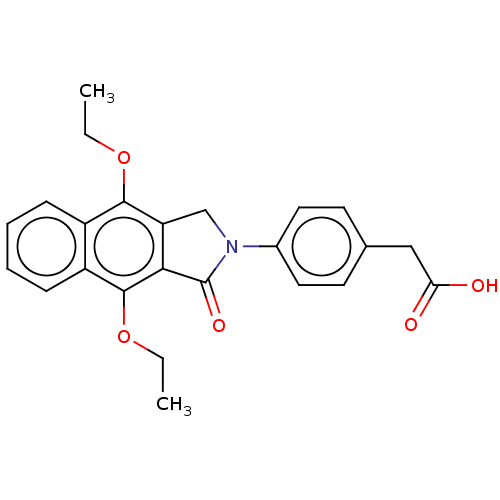
(CHEMBL4225786)Show SMILES CCOc1c2CN(C(=O)c2c(OCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C24H23NO5/c1-3-29-22-17-7-5-6-8-18(17)23(30-4-2)21-19(22)14-25(24(21)28)16-11-9-15(10-12-16)13-20(26)27/h5-12H,3-4,13-14H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461454

(CHEMBL4224936)Show SMILES CCOc1c2C(=O)N(C(=O)c2c(OCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C24H21NO6/c1-3-30-21-16-7-5-6-8-17(16)22(31-4-2)20-19(21)23(28)25(24(20)29)15-11-9-14(10-12-15)13-18(26)27/h5-12H,3-4,13H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461457
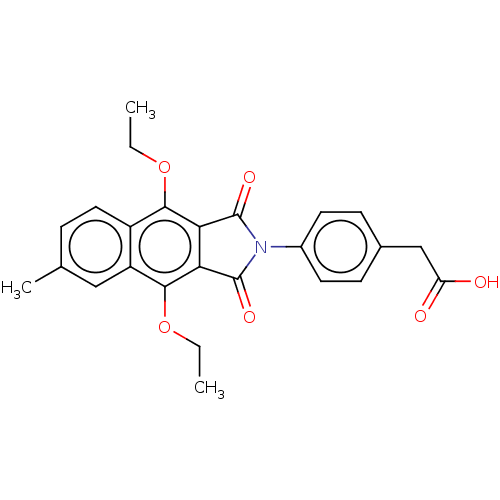
(CHEMBL4225963)Show SMILES CCOc1c2C(=O)N(C(=O)c2c(OCC)c2cc(C)ccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C25H23NO6/c1-4-31-22-17-11-6-14(3)12-18(17)23(32-5-2)21-20(22)24(29)26(25(21)30)16-9-7-15(8-10-16)13-19(27)28/h6-12H,4-5,13H2,1-3H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461450

(CHEMBL4226720)Show SMILES CCOc1c2CN(C(=O)c2c(OCC)c2ccccc12)c1ccc(cc1)C(C)C(O)=O Show InChI InChI=1S/C25H25NO5/c1-4-30-22-18-8-6-7-9-19(18)23(31-5-2)21-20(22)14-26(24(21)27)17-12-10-16(11-13-17)15(3)25(28)29/h6-13,15H,4-5,14H2,1-3H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461451

(CHEMBL4226523)Show SMILES CC(C)Oc1c2CN(C(=O)c2c(OC(C)C)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C26H27NO5/c1-15(2)31-24-19-7-5-6-8-20(19)25(32-16(3)4)23-21(24)14-27(26(23)30)18-11-9-17(10-12-18)13-22(28)29/h5-12,15-16H,13-14H2,1-4H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461463

(CHEMBL4225442)Show SMILES CCCOc1c2CN(C(=O)c2c(OCCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C26H27NO5/c1-3-13-31-24-19-7-5-6-8-20(19)25(32-14-4-2)23-21(24)16-27(26(23)30)18-11-9-17(10-12-18)15-22(28)29/h5-12H,3-4,13-16H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461453
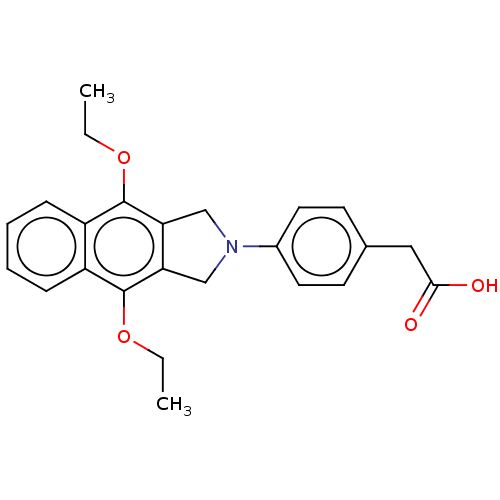
(CHEMBL4226984)Show SMILES CCOc1c2CN(Cc2c(OCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C24H25NO4/c1-3-28-23-18-7-5-6-8-19(18)24(29-4-2)21-15-25(14-20(21)23)17-11-9-16(10-12-17)13-22(26)27/h5-12H,3-4,13-15H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(RAT) | BDBM50302272
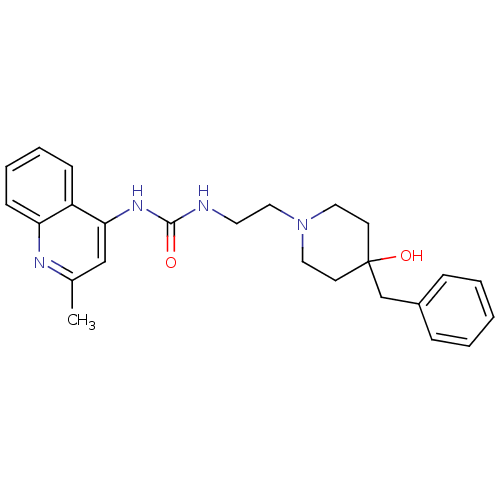
(1-(2-(4-benzyl-4-hydroxypiperidin-1-yl)ethyl)-3-(2...)Show SMILES Cc1cc(NC(=O)NCCN2CCC(O)(Cc3ccccc3)CC2)c2ccccc2n1 Show InChI InChI=1S/C25H30N4O2/c1-19-17-23(21-9-5-6-10-22(21)27-19)28-24(30)26-13-16-29-14-11-25(31,12-15-29)18-20-7-3-2-4-8-20/h2-10,17,31H,11-16,18H2,1H3,(H2,26,27,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 311: 204-12 (2004)
Article DOI: 10.1124/jpet.104.068320
BindingDB Entry DOI: 10.7270/Q2H993RW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461468
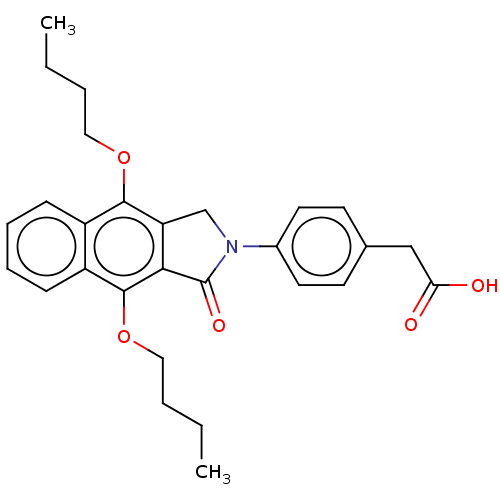
(CHEMBL4227243)Show SMILES CCCCOc1c2CN(C(=O)c2c(OCCCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C28H31NO5/c1-3-5-15-33-26-21-9-7-8-10-22(21)27(34-16-6-4-2)25-23(26)18-29(28(25)32)20-13-11-19(12-14-20)17-24(30)31/h7-14H,3-6,15-18H2,1-2H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50461456

(CHEMBL4228095)Show SMILES CCCCCCOc1c2CN(C(=O)c2c(OCCCCCC)c2ccccc12)c1ccc(CC(O)=O)cc1 Show InChI InChI=1S/C32H39NO5/c1-3-5-7-11-19-37-30-25-13-9-10-14-26(25)31(38-20-12-8-6-4-2)29-27(30)22-33(32(29)36)24-17-15-23(16-18-24)21-28(34)35/h9-10,13-18H,3-8,11-12,19-22H2,1-2H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| <6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK 293 (EBNA) cell membranes incubated for 60 mins by scintillation counting method |
Bioorg Med Chem Lett 28: 1892-1896 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.091
BindingDB Entry DOI: 10.7270/Q21Z472W |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50330345
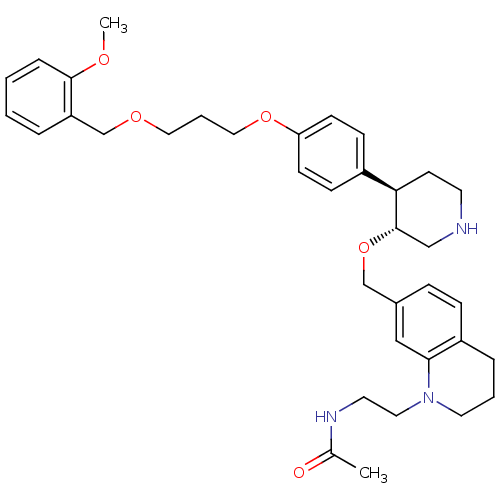
(CHEMBL1276275 | N-(2-(7-(((3R,4R)-4-(4-(3-(2-metho...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)[C@H]1CCNC[C@@H]1OCc1ccc2CCCN(CCNC(C)=O)c2c1 |r| Show InChI InChI=1S/C36H47N3O5/c1-27(40)38-18-20-39-19-5-8-30-11-10-28(23-34(30)39)25-44-36-24-37-17-16-33(36)29-12-14-32(15-13-29)43-22-6-21-42-26-31-7-3-4-9-35(31)41-2/h3-4,7,9-15,23,33,36-37H,5-6,8,16-22,24-26H2,1-2H3,(H,38,40)/t33-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay |
J Med Chem 52: 3689-702 (2009)
Article DOI: 10.1021/jm900022f
BindingDB Entry DOI: 10.7270/Q2PC3391 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328867
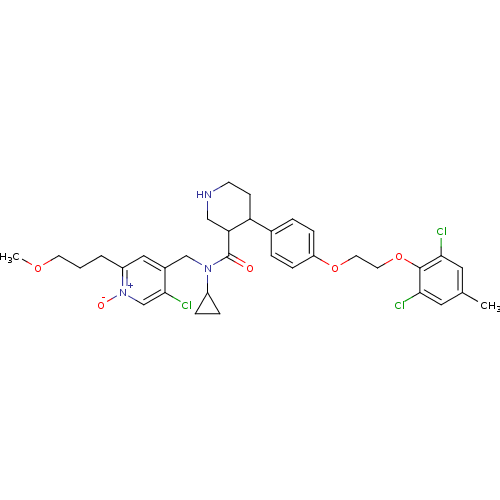
(CHEMBL1269698 | rac-5-chloro-4-((N-cyclopropyl-4-(...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)C2CNCCC2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c(Cl)c[n+]1[O-] Show InChI InChI=1S/C34H40Cl3N3O5/c1-22-16-30(35)33(31(36)17-22)45-15-14-44-27-9-5-23(6-10-27)28-11-12-38-19-29(28)34(41)39(25-7-8-25)20-24-18-26(4-3-13-43-2)40(42)21-32(24)37/h5-6,9-10,16-18,21,25,28-29,38H,3-4,7-8,11-15,19-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328846
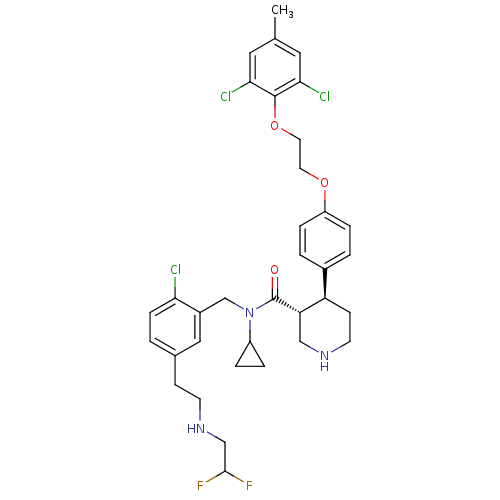
((3R,4S)-N-(2-chloro-5-(2-(2,2-difluoroethylamino)e...)Show SMILES Cc1cc(Cl)c(OCCOc2ccc(cc2)[C@H]2CCNC[C@@H]2C(=O)N(Cc2cc(CCNCC(F)F)ccc2Cl)C2CC2)c(Cl)c1 |r| Show InChI InChI=1S/C35H40Cl3F2N3O3/c1-22-16-31(37)34(32(38)17-22)46-15-14-45-27-7-3-24(4-8-27)28-11-13-41-19-29(28)35(44)43(26-5-6-26)21-25-18-23(2-9-30(25)36)10-12-42-20-33(39)40/h2-4,7-9,16-18,26,28-29,33,41-42H,5-6,10-15,19-21H2,1H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328916
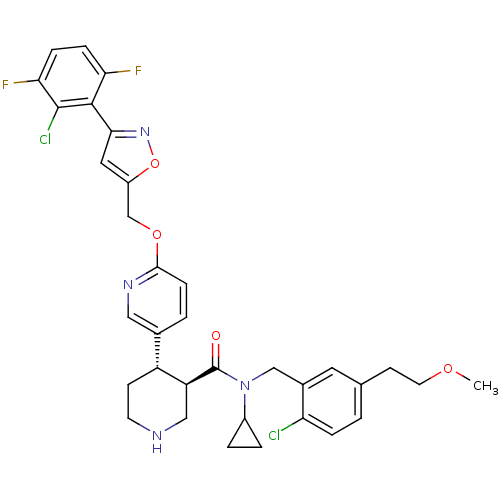
((3R,4S)-4-(6-((3-(2-chloro-3,6-difluorophenyl)isox...)Show SMILES COCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCc3cc(no3)-c3c(F)ccc(F)c3Cl)nc2)c1 |r| Show InChI InChI=1S/C34H34Cl2F2N4O4/c1-44-13-11-20-2-6-27(35)22(14-20)18-42(23-4-5-23)34(43)26-17-39-12-10-25(26)21-3-9-31(40-16-21)45-19-24-15-30(41-46-24)32-28(37)7-8-29(38)33(32)36/h2-3,6-9,14-16,23,25-26,39H,4-5,10-13,17-19H2,1H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328908

(CHEMBL1269746 | methyl 4-chloro-3-(((3R,4S)-N-cycl...)Show SMILES COC(=O)NCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)nc2)c1 |r| Show InChI InChI=1S/C33H37Cl3N4O5/c1-20-13-28(35)31(29(36)14-20)45-12-11-44-30-8-4-22(17-38-30)25-9-10-37-18-26(25)32(41)40(24-5-6-24)19-23-15-21(3-7-27(23)34)16-39-33(42)43-2/h3-4,7-8,13-15,17,24-26,37H,5-6,9-12,16,18-19H2,1-2H3,(H,39,42)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328905
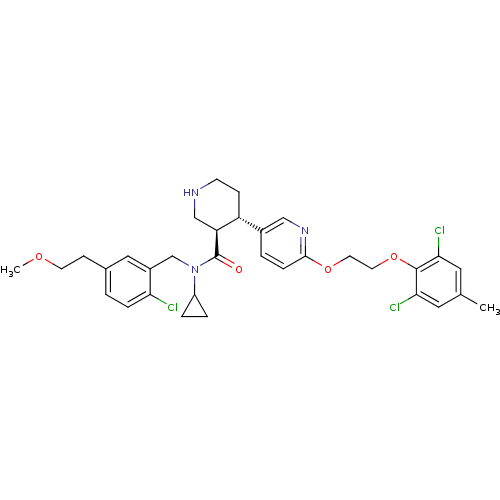
((3R,4S)-N-(2-chloro-5-(2-methoxyethyl)benzyl)-N-cy...)Show SMILES COCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)nc2)c1 |r| Show InChI InChI=1S/C33H38Cl3N3O4/c1-21-15-29(35)32(30(36)16-21)43-14-13-42-31-8-4-23(18-38-31)26-9-11-37-19-27(26)33(40)39(25-5-6-25)20-24-17-22(10-12-41-2)3-7-28(24)34/h3-4,7-8,15-18,25-27,37H,5-6,9-14,19-20H2,1-2H3/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328910
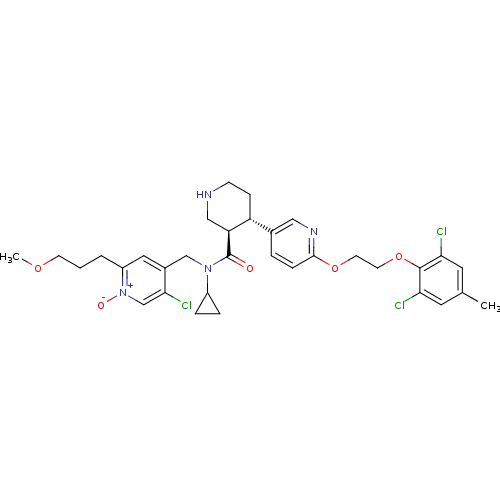
(5-chloro-4-(((3R,4S)-N-cyclopropyl-4-(6-(2-(2,6-di...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)nc2)c(Cl)c[n+]1[O-] |r| Show InChI InChI=1S/C33H39Cl3N4O5/c1-21-14-28(34)32(29(35)15-21)45-13-12-44-31-8-5-22(17-38-31)26-9-10-37-18-27(26)33(41)39(24-6-7-24)19-23-16-25(4-3-11-43-2)40(42)20-30(23)36/h5,8,14-17,20,24,26-27,37H,3-4,6-7,9-13,18-19H2,1-2H3/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328906
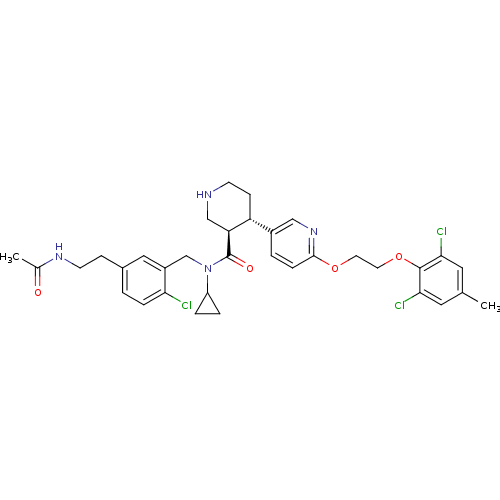
((3R,4S)-N-(5-(2-acetamidoethyl)-2-chlorobenzyl)-N-...)Show SMILES CC(=O)NCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)nc2)c1 |r| Show InChI InChI=1S/C34H39Cl3N4O4/c1-21-15-30(36)33(31(37)16-21)45-14-13-44-32-8-4-24(18-40-32)27-10-11-38-19-28(27)34(43)41(26-5-6-26)20-25-17-23(3-7-29(25)35)9-12-39-22(2)42/h3-4,7-8,15-18,26-28,38H,5-6,9-14,19-20H2,1-2H3,(H,39,42)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328911
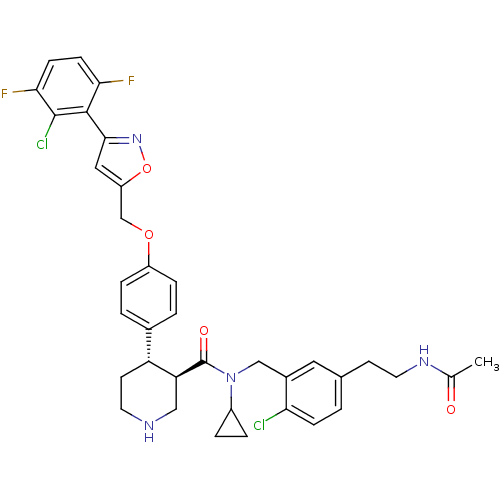
((3R,4S)-N-(5-(2-acetamidoethyl)-2-chlorobenzyl)-4-...)Show SMILES CC(=O)NCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCc3cc(no3)-c3c(F)ccc(F)c3Cl)cc2)c1 |r| Show InChI InChI=1S/C36H36Cl2F2N4O4/c1-21(45)42-15-12-22-2-9-30(37)24(16-22)19-44(25-5-6-25)36(46)29-18-41-14-13-28(29)23-3-7-26(8-4-23)47-20-27-17-33(43-48-27)34-31(39)10-11-32(40)35(34)38/h2-4,7-11,16-17,25,28-29,41H,5-6,12-15,18-20H2,1H3,(H,42,45)/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328861
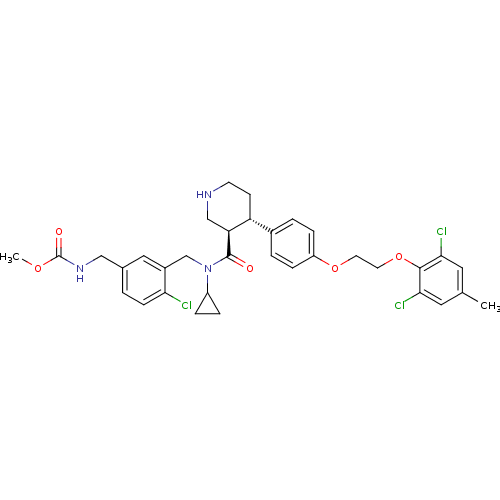
(CHEMBL1269696 | methyl 4-chloro-3-(((3R,4S)-N-cycl...)Show SMILES COC(=O)NCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C34H38Cl3N3O5/c1-21-15-30(36)32(31(37)16-21)45-14-13-44-26-8-4-23(5-9-26)27-11-12-38-19-28(27)33(41)40(25-6-7-25)20-24-17-22(3-10-29(24)35)18-39-34(42)43-2/h3-5,8-10,15-17,25,27-28,38H,6-7,11-14,18-20H2,1-2H3,(H,39,42)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328861

(CHEMBL1269696 | methyl 4-chloro-3-(((3R,4S)-N-cycl...)Show SMILES COC(=O)NCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C34H38Cl3N3O5/c1-21-15-30(36)32(31(37)16-21)45-14-13-44-26-8-4-23(5-9-26)27-11-12-38-19-28(27)33(41)40(25-6-7-25)20-24-17-22(3-10-29(24)35)18-39-34(42)43-2/h3-5,8-10,15-17,25,27-28,38H,6-7,11-14,18-20H2,1-2H3,(H,39,42)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328923
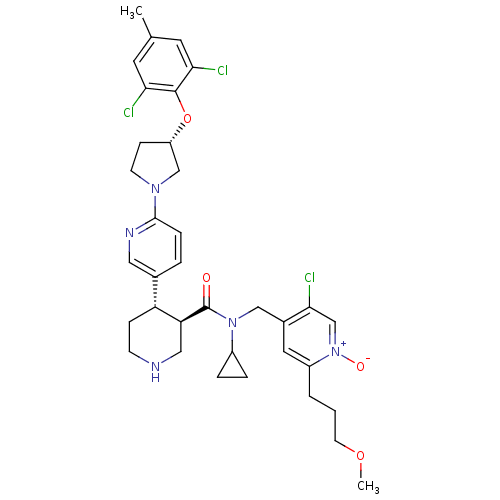
(5-chloro-4-(((3R,4S)-N-cyclopropyl-4-(6-((S)-3-(2,...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(nc2)N2CC[C@@H](C2)Oc2c(Cl)cc(C)cc2Cl)c(Cl)c[n+]1[O-] |r| Show InChI InChI=1S/C35H42Cl3N5O4/c1-22-14-30(36)34(31(37)15-22)47-27-10-12-41(20-27)33-8-5-23(17-40-33)28-9-11-39-18-29(28)35(44)42(25-6-7-25)19-24-16-26(4-3-13-46-2)43(45)21-32(24)38/h5,8,14-17,21,25,27-29,39H,3-4,6-7,9-13,18-20H2,1-2H3/t27-,28+,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328922
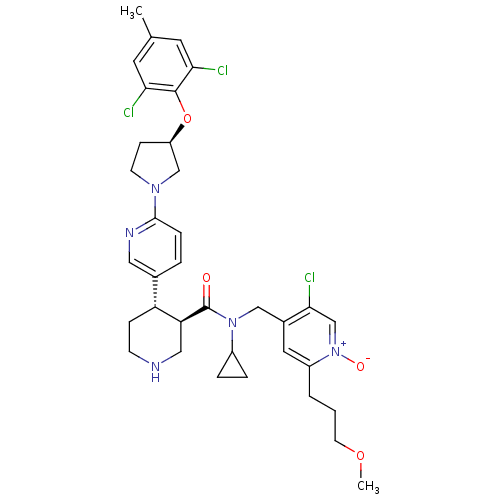
(5-chloro-4-(((3R,4S)-N-cyclopropyl-4-(6-((R)-3-(2,...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(nc2)N2CC[C@H](C2)Oc2c(Cl)cc(C)cc2Cl)c(Cl)c[n+]1[O-] |r| Show InChI InChI=1S/C35H42Cl3N5O4/c1-22-14-30(36)34(31(37)15-22)47-27-10-12-41(20-27)33-8-5-23(17-40-33)28-9-11-39-18-29(28)35(44)42(25-6-7-25)19-24-16-26(4-3-13-46-2)43(45)21-32(24)38/h5,8,14-17,21,25,27-29,39H,3-4,6-7,9-13,18-20H2,1-2H3/t27-,28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328856
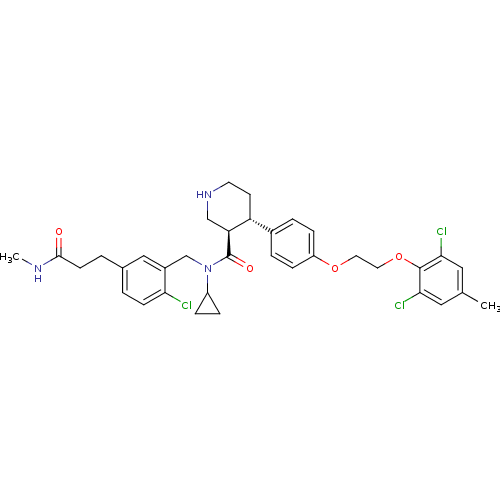
((3R,4S)-N-(2-chloro-5-(3-(methylamino)-3-oxopropyl...)Show SMILES CNC(=O)CCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C35H40Cl3N3O4/c1-22-17-31(37)34(32(38)18-22)45-16-15-44-27-9-5-24(6-10-27)28-13-14-40-20-29(28)35(43)41(26-7-8-26)21-25-19-23(3-11-30(25)36)4-12-33(42)39-2/h3,5-6,9-11,17-19,26,28-29,40H,4,7-8,12-16,20-21H2,1-2H3,(H,39,42)/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328919
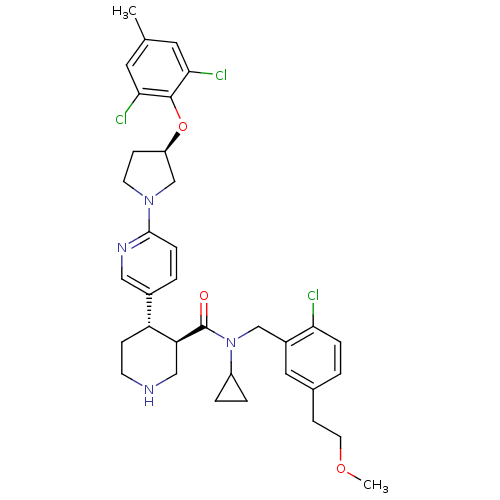
((3R,4S)-N-(2-chloro-5-(2-methoxyethyl)benzyl)-N-cy...)Show SMILES COCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(nc2)N2CC[C@H](C2)Oc2c(Cl)cc(C)cc2Cl)c1 |r| Show InChI InChI=1S/C35H41Cl3N4O3/c1-22-15-31(37)34(32(38)16-22)45-27-10-13-41(21-27)33-8-4-24(18-40-33)28-9-12-39-19-29(28)35(43)42(26-5-6-26)20-25-17-23(11-14-44-2)3-7-30(25)36/h3-4,7-8,15-18,26-29,39H,5-6,9-14,19-21H2,1-2H3/t27-,28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328915
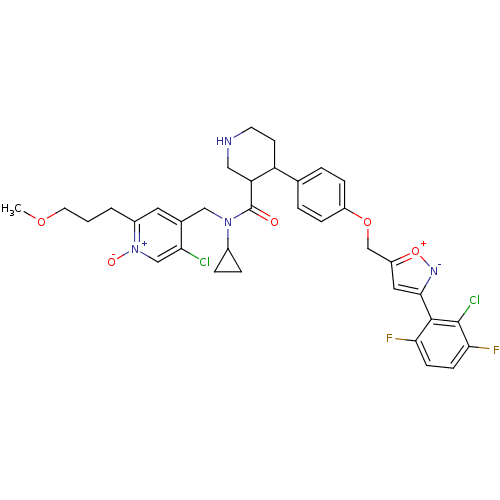
(5-chloro-4-(((3R,4S)-4-(4-((3-(2-chloro-3,6-difluo...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)C2CNCCC2c2ccc(OCc3cc([n-][o+]3)-c3c(F)ccc(F)c3Cl)cc2)c(Cl)c[n+]1[O-] Show InChI InChI=1S/C35H36Cl2F2N4O5/c1-46-14-2-3-24-15-22(29(36)19-43(24)45)18-42(23-6-7-23)35(44)28-17-40-13-12-27(28)21-4-8-25(9-5-21)47-20-26-16-32(41-48-26)33-30(38)10-11-31(39)34(33)37/h4-5,8-11,15-16,19,23,27-28,40H,2-3,6-7,12-14,17-18,20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328924
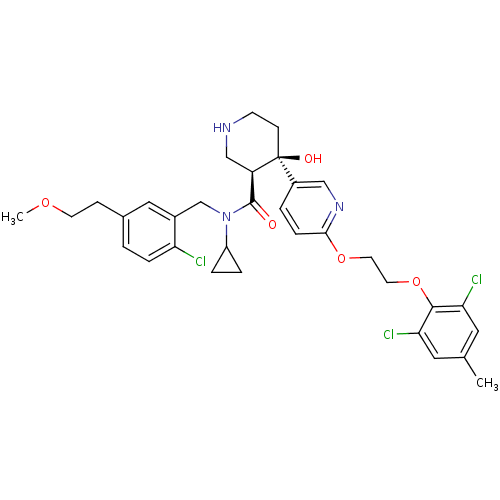
((3S,4R)-N-(2-chloro-5-(2-methoxyethyl)benzyl)-N-cy...)Show SMILES COCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@]2(O)c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)nc2)c1 |r| Show InChI InChI=1S/C33H38Cl3N3O5/c1-21-15-28(35)31(29(36)16-21)44-14-13-43-30-8-4-24(18-38-30)33(41)10-11-37-19-26(33)32(40)39(25-5-6-25)20-23-17-22(9-12-42-2)3-7-27(23)34/h3-4,7-8,15-18,25-26,37,41H,5-6,9-14,19-20H2,1-2H3/t26-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50328917
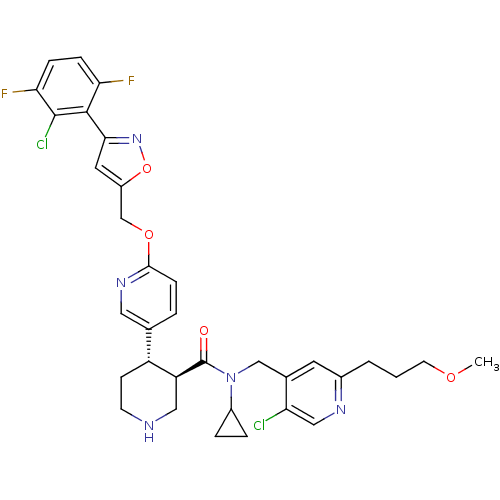
((3R,4S)-N-((5-chloro-2-(3-methoxypropyl)pyridin-4-...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCc3cc(no3)-c3c(F)ccc(F)c3Cl)nc2)c(Cl)cn1 |r| Show InChI InChI=1S/C34H35Cl2F2N5O4/c1-45-12-2-3-22-13-21(27(35)17-40-22)18-43(23-5-6-23)34(44)26-16-39-11-10-25(26)20-4-9-31(41-15-20)46-19-24-14-30(42-47-24)32-28(37)7-8-29(38)33(32)36/h4,7-9,13-15,17,23,25-26,39H,2-3,5-6,10-12,16,18-19H2,1H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328909
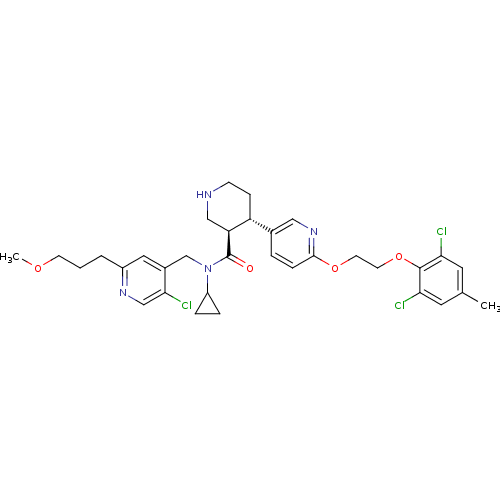
((3R,4S)-N-((5-chloro-2-(3-methoxypropyl)pyridin-4-...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)nc2)c(Cl)cn1 |r| Show InChI InChI=1S/C33H39Cl3N4O4/c1-21-14-28(34)32(29(35)15-21)44-13-12-43-31-8-5-22(17-39-31)26-9-10-37-18-27(26)33(41)40(25-6-7-25)20-23-16-24(4-3-11-42-2)38-19-30(23)36/h5,8,14-17,19,25-27,37H,3-4,6-7,9-13,18,20H2,1-2H3/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328844
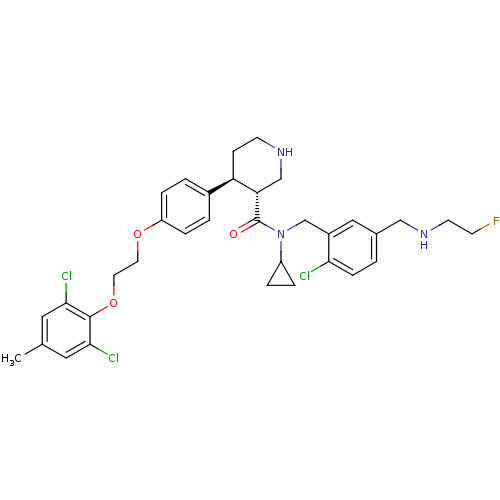
((3R,4S)-N-(2-chloro-5-((2-fluoroethylamino)methyl)...)Show SMILES Cc1cc(Cl)c(OCCOc2ccc(cc2)[C@H]2CCNC[C@@H]2C(=O)N(Cc2cc(CNCCF)ccc2Cl)C2CC2)c(Cl)c1 |r| Show InChI InChI=1S/C34H39Cl3FN3O3/c1-22-16-31(36)33(32(37)17-22)44-15-14-43-27-7-3-24(4-8-27)28-10-12-39-20-29(28)34(42)41(26-5-6-26)21-25-18-23(2-9-30(25)35)19-40-13-11-38/h2-4,7-9,16-18,26,28-29,39-40H,5-6,10-15,19-21H2,1H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328926
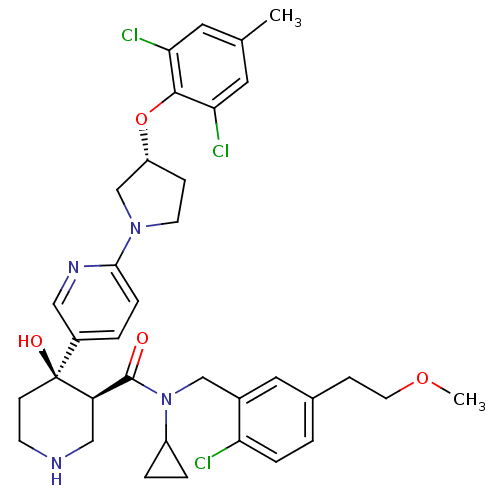
((3S,4R)-N-(2-chloro-5-(2-methoxyethyl)benzyl)-N-cy...)Show SMILES COCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@]2(O)c2ccc(nc2)N2CC[C@H](C2)Oc2c(Cl)cc(C)cc2Cl)c1 |r| Show InChI InChI=1S/C35H41Cl3N4O4/c1-22-15-30(37)33(31(38)16-22)46-27-9-13-41(21-27)32-8-4-25(18-40-32)35(44)11-12-39-19-28(35)34(43)42(26-5-6-26)20-24-17-23(10-14-45-2)3-7-29(24)36/h3-4,7-8,15-18,26-28,39,44H,5-6,9-14,19-21H2,1-2H3/t27-,28-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328913
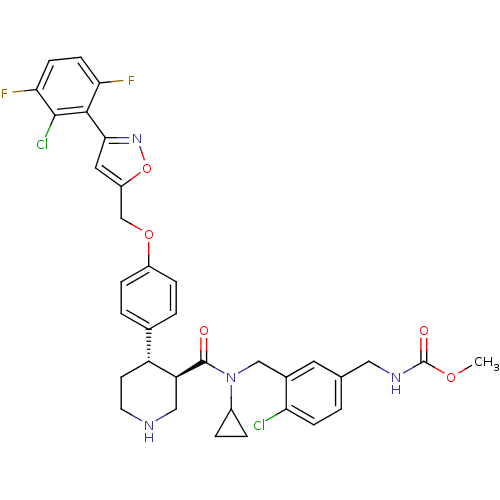
(CHEMBL1269751 | methyl 4-chloro-3-(((3R,4S)-4-(4-(...)Show SMILES COC(=O)NCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCc3cc(no3)-c3c(F)ccc(F)c3Cl)cc2)c1 |r| Show InChI InChI=1S/C35H34Cl2F2N4O5/c1-46-35(45)41-16-20-2-9-28(36)22(14-20)18-43(23-5-6-23)34(44)27-17-40-13-12-26(27)21-3-7-24(8-4-21)47-19-25-15-31(42-48-25)32-29(38)10-11-30(39)33(32)37/h2-4,7-11,14-15,23,26-27,40H,5-6,12-13,16-19H2,1H3,(H,41,45)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328904
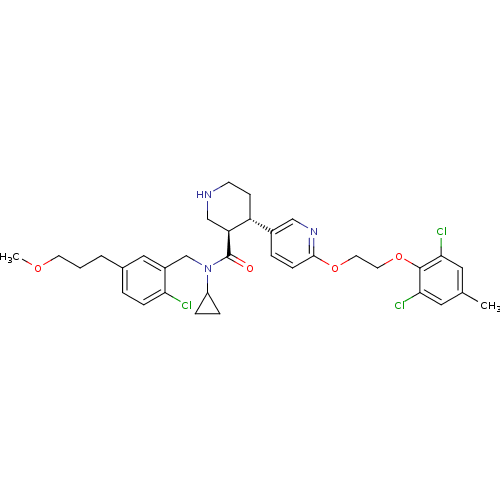
((3R,4S)-N-(2-chloro-5-(3-methoxypropyl)benzyl)-N-c...)Show SMILES COCCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)nc2)c1 |r| Show InChI InChI=1S/C34H40Cl3N3O4/c1-22-16-30(36)33(31(37)17-22)44-15-14-43-32-10-6-24(19-39-32)27-11-12-38-20-28(27)34(41)40(26-7-8-26)21-25-18-23(4-3-13-42-2)5-9-29(25)35/h5-6,9-10,16-19,26-28,38H,3-4,7-8,11-15,20-21H2,1-2H3/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50259452
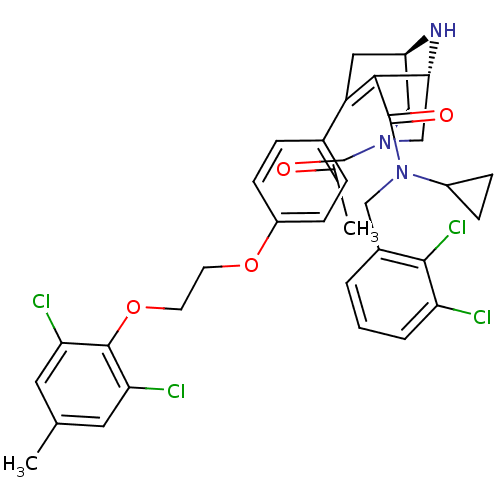
((1R,5S)-3-Acetyl-7-{4-[2-(2,6-dichloro-4-methyl-ph...)Show SMILES [H][C@@]12CN(C[C@@]([H])(N1)C(C(=O)N(Cc1cccc(Cl)c1Cl)C1CC1)=C(C2)c1ccc(OCCOc2c(Cl)cc(C)cc2Cl)cc1)C(C)=O |r,wU:1.0,wD:5.5,c:26,TLB:26:24:7:3.4.2,THB:45:3:7:8.24.25,9:8:7:3.4.2,(12.06,-12.12,;11.66,-10.63,;13.38,-10.97,;13.92,-9.5,;12.81,-10.12,;11,-9.79,;9.51,-10.19,;10.46,-11.26,;10.28,-8.29,;8.74,-8.23,;8.02,-6.87,;7.92,-9.53,;6.38,-9.48,;5.57,-10.78,;6.29,-12.13,;5.48,-13.44,;3.94,-13.39,;3.21,-12.02,;1.68,-11.97,;4.03,-10.72,;3.31,-9.36,;8.64,-10.89,;8.59,-12.43,;9.95,-11.71,;11.4,-7.93,;11.93,-8.91,;12.23,-7.52,;13.77,-7.53,;14.54,-6.19,;13.76,-4.86,;14.53,-3.52,;16.07,-3.51,;16.83,-2.18,;18.37,-2.17,;19.14,-.83,;18.37,.48,;16.83,.48,;19.12,1.81,;20.67,1.83,;21.43,3.17,;21.45,.5,;20.68,-.83,;21.45,-2.17,;12.22,-4.87,;11.46,-6.2,;15.22,-8.67,;16.58,-9.39,;15.16,-7.14,)| Show InChI InChI=1S/C35H35Cl4N3O4/c1-20-14-29(37)34(30(38)15-20)46-13-12-45-26-10-6-22(7-11-26)27-16-24-18-41(21(2)43)19-31(40-24)32(27)35(44)42(25-8-9-25)17-23-4-3-5-28(36)33(23)39/h3-7,10-11,14-15,24-25,31,40H,8-9,12-13,16-19H2,1-2H3/t24-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay |
J Med Chem 52: 3689-702 (2009)
Article DOI: 10.1021/jm900022f
BindingDB Entry DOI: 10.7270/Q2PC3391 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328907
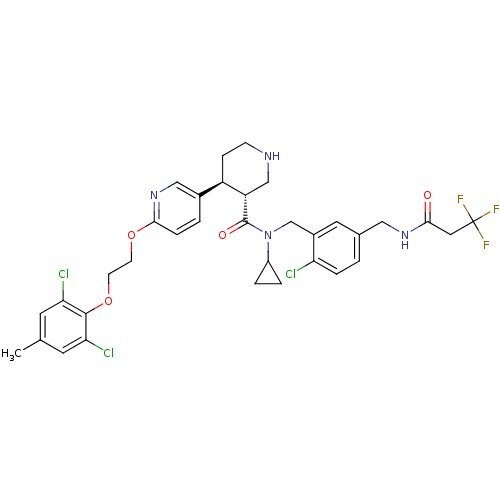
((3R,4S)-N-(2-chloro-5-((3,3,3-trifluoropropanamido...)Show SMILES Cc1cc(Cl)c(OCCOc2ccc(cn2)[C@H]2CCNC[C@@H]2C(=O)N(Cc2cc(CNC(=O)CC(F)(F)F)ccc2Cl)C2CC2)c(Cl)c1 |r| Show InChI InChI=1S/C34H36Cl3F3N4O4/c1-20-12-28(36)32(29(37)13-20)48-11-10-47-31-7-3-22(17-43-31)25-8-9-41-18-26(25)33(46)44(24-4-5-24)19-23-14-21(2-6-27(23)35)16-42-30(45)15-34(38,39)40/h2-3,6-7,12-14,17,24-26,41H,4-5,8-11,15-16,18-19H2,1H3,(H,42,45)/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328914
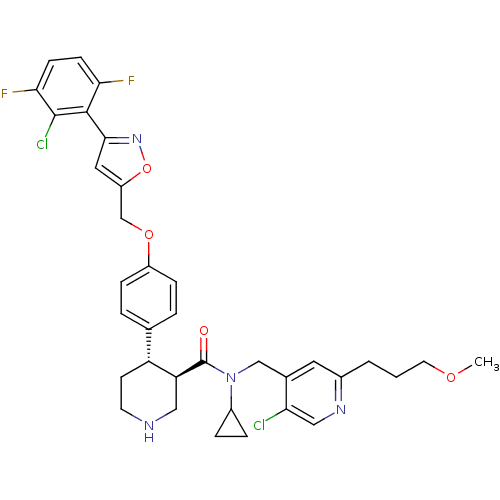
((3R,4S)-N-((5-chloro-2-(3-methoxypropyl)pyridin-4-...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCc3cc(no3)-c3c(F)ccc(F)c3Cl)cc2)c(Cl)cn1 |r| Show InChI InChI=1S/C35H36Cl2F2N4O4/c1-45-14-2-3-23-15-22(29(36)18-41-23)19-43(24-6-7-24)35(44)28-17-40-13-12-27(28)21-4-8-25(9-5-21)46-20-26-16-32(42-47-26)33-30(38)10-11-31(39)34(33)37/h4-5,8-11,15-16,18,24,27-28,40H,2-3,6-7,12-14,17,19-20H2,1H3/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328912
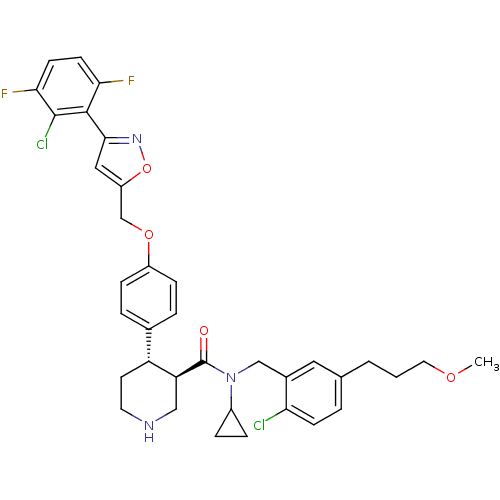
((3R,4S)-4-(4-((3-(2-chloro-3,6-difluorophenyl)isox...)Show SMILES COCCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCc3cc(no3)-c3c(F)ccc(F)c3Cl)cc2)c1 |r| Show InChI InChI=1S/C36H37Cl2F2N3O4/c1-45-16-2-3-22-4-11-30(37)24(17-22)20-43(25-7-8-25)36(44)29-19-41-15-14-28(29)23-5-9-26(10-6-23)46-21-27-18-33(42-47-27)34-31(39)12-13-32(40)35(34)38/h4-6,9-13,17-18,25,28-29,41H,2-3,7-8,14-16,19-21H2,1H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
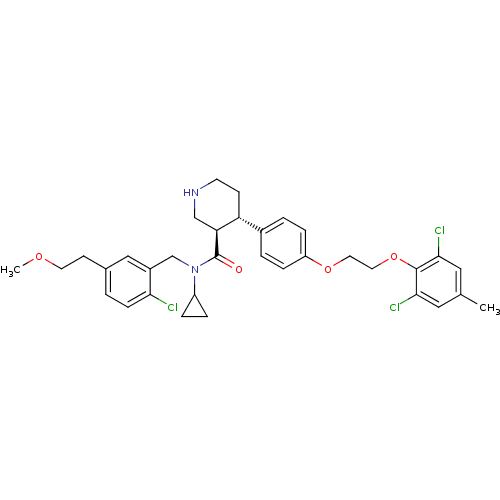
(Homo sapiens (Human)) | BDBM50328852
((3R,4S)-N-(2-chloro-5-(2-methoxyethyl)benzyl)-N-cy...)Show SMILES COCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C34H39Cl3N2O4/c1-22-17-31(36)33(32(37)18-22)43-16-15-42-27-8-4-24(5-9-27)28-11-13-38-20-29(28)34(40)39(26-6-7-26)21-25-19-23(12-14-41-2)3-10-30(25)35/h3-5,8-10,17-19,26,28-29,38H,6-7,11-16,20-21H2,1-2H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
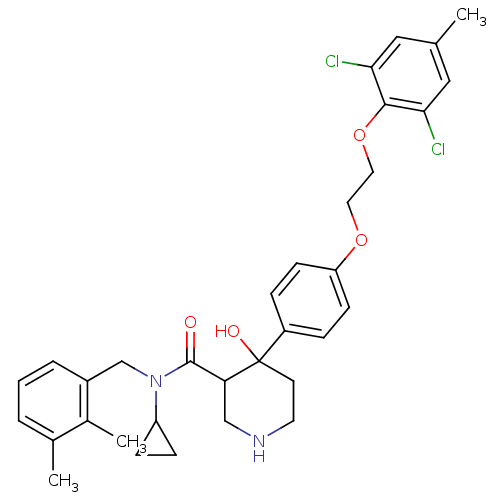
(Homo sapiens (Human)) | BDBM50328866
(CHEMBL1270761 | rac-N-cyclopropyl-4-(4-(2-(2,6-dic...)Show SMILES Cc1cc(Cl)c(OCCOc2ccc(cc2)C2(O)CCNCC2C(=O)N(Cc2cccc(C)c2C)C2CC2)c(Cl)c1 Show InChI InChI=1S/C33H38Cl2N2O4/c1-21-17-29(34)31(30(35)18-21)41-16-15-40-27-11-7-25(8-12-27)33(39)13-14-36-19-28(33)32(38)37(26-9-10-26)20-24-6-4-5-22(2)23(24)3/h4-8,11-12,17-18,26,28,36,39H,9-10,13-16,19-20H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328852

((3R,4S)-N-(2-chloro-5-(2-methoxyethyl)benzyl)-N-cy...)Show SMILES COCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C34H39Cl3N2O4/c1-22-17-31(36)33(32(37)18-22)43-16-15-42-27-8-4-24(5-9-27)28-11-13-38-20-29(28)34(40)39(26-6-7-26)21-25-19-23(12-14-41-2)3-10-30(25)35/h3-5,8-10,17-19,26,28-29,38H,6-7,11-16,20-21H2,1-2H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328852

((3R,4S)-N-(2-chloro-5-(2-methoxyethyl)benzyl)-N-cy...)Show SMILES COCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C34H39Cl3N2O4/c1-22-17-31(36)33(32(37)18-22)43-16-15-42-27-8-4-24(5-9-27)28-11-13-38-20-29(28)34(40)39(26-6-7-26)21-25-19-23(12-14-41-2)3-10-30(25)35/h3-5,8-10,17-19,26,28-29,38H,6-7,11-16,20-21H2,1-2H3/t28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
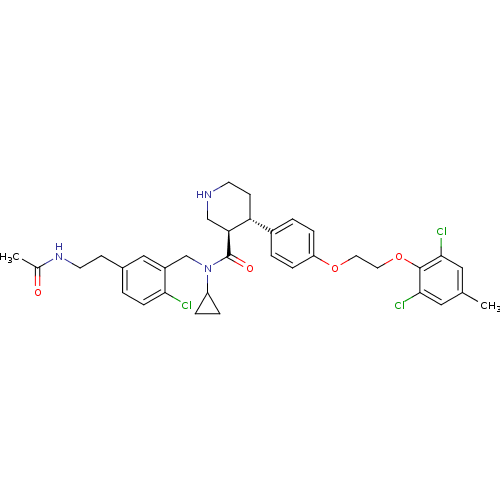
(Homo sapiens (Human)) | BDBM50328855
((3R,4S)-N-(5-(2-acetamidoethyl)-2-chlorobenzyl)-N-...)Show SMILES CC(=O)NCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C35H40Cl3N3O4/c1-22-17-32(37)34(33(38)18-22)45-16-15-44-28-8-4-25(5-9-28)29-12-13-39-20-30(29)35(43)41(27-6-7-27)21-26-19-24(3-10-31(26)36)11-14-40-23(2)42/h3-5,8-10,17-19,27,29-30,39H,6-7,11-16,20-21H2,1-2H3,(H,40,42)/t29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50328855

((3R,4S)-N-(5-(2-acetamidoethyl)-2-chlorobenzyl)-N-...)Show SMILES CC(=O)NCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C35H40Cl3N3O4/c1-22-17-32(37)34(33(38)18-22)45-16-15-44-28-8-4-25(5-9-28)29-12-13-39-20-30(29)35(43)41(27-6-7-27)21-26-19-24(3-10-31(26)36)11-14-40-23(2)42/h3-5,8-10,17-19,27,29-30,39H,6-7,11-16,20-21H2,1-2H3,(H,40,42)/t29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6286-90 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.086
BindingDB Entry DOI: 10.7270/Q2WD40T3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
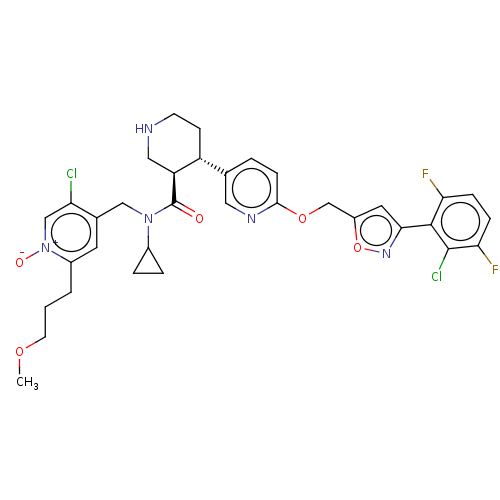
(Homo sapiens (Human)) | BDBM50328918
(5-chloro-4-(((3R,4S)-4-(6-((3-(2-chloro-3,6-difluo...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCc3cc(no3)-c3c(F)ccc(F)c3Cl)nc2)c(Cl)c[n+]1[O-] |r| Show InChI InChI=1S/C34H35Cl2F2N5O5/c1-46-12-2-3-23-13-21(27(35)18-43(23)45)17-42(22-5-6-22)34(44)26-16-39-11-10-25(26)20-4-9-31(40-15-20)47-19-24-14-30(41-48-24)32-28(37)7-8-29(38)33(32)36/h4,7-9,13-15,18,22,25-26,39H,2-3,5-6,10-12,16-17,19H2,1H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in plasma |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50259432

((1R,5S)-3-Acetyl-7-{4-[3-(2-methoxy-benzyloxy)-pro...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)C1=C([C@H]2CN(C[C@@H](C1)N2)C(C)=O)C(=O)N(Cc1cccc(Cl)c1Cl)C1CC1 |r,wU:22.31,wD:26.30,t:22,TLB:17:20:28:24.23.25,29:24:28:21.20.27,THB:32:21:28:24.23.25,(20.64,-1.86,;21.41,-.53,;20.63,.81,;21.4,2.14,;20.62,3.47,;19.07,3.45,;18.32,2.12,;19.09,.81,;18.32,-.53,;16.78,-.54,;16.02,-1.87,;14.48,-1.88,;13.71,-3.22,;12.17,-3.23,;11.41,-4.56,;12.18,-5.9,;11.42,-7.23,;9.88,-7.23,;9.1,-5.91,;9.86,-4.57,;9.2,-7.98,;7.92,-7.99,;8.64,-9.5,;10.45,-9.83,;11.56,-9.2,;11.02,-10.68,;9.3,-10.34,;9.6,-8.91,;8.11,-10.96,;12.86,-8.38,;14.23,-9.1,;12.8,-6.84,;6.38,-7.93,;5.66,-6.57,;5.57,-9.24,;4.03,-9.18,;3.21,-10.49,;3.94,-11.84,;3.13,-13.14,;1.58,-13.09,;.86,-11.73,;-.68,-11.67,;1.68,-10.43,;.96,-9.06,;6.29,-10.6,;6.24,-12.14,;7.6,-11.42,)| Show InChI InChI=1S/C37H41Cl2N3O5/c1-24(43)41-21-28-19-31(25-11-15-30(16-12-25)47-18-6-17-46-23-27-7-3-4-10-34(27)45-2)35(33(22-41)40-28)37(44)42(29-13-14-29)20-26-8-5-9-32(38)36(26)39/h3-5,7-12,15-16,28-29,33,40H,6,13-14,17-23H2,1-2H3/t28-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay |
J Med Chem 52: 3689-702 (2009)
Article DOI: 10.1021/jm900022f
BindingDB Entry DOI: 10.7270/Q2PC3391 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50328925
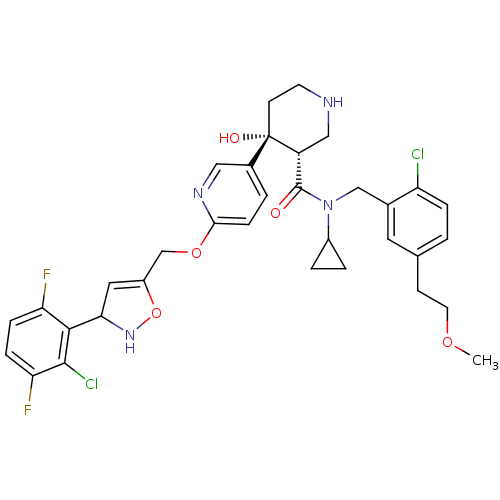
((3S,4R)-4-(6-((3-(2-chloro-3,6-difluorophenyl)-2,3...)Show SMILES COCCc1ccc(Cl)c(CN(C2CC2)C(=O)[C@H]2CNCC[C@]2(O)c2ccc(OCC3=CC(NO3)c3c(F)ccc(F)c3Cl)nc2)c1 |r,t:32| Show InChI InChI=1S/C34H36Cl2F2N4O5/c1-45-13-10-20-2-6-26(35)21(14-20)18-42(23-4-5-23)33(43)25-17-39-12-11-34(25,44)22-3-9-30(40-16-22)46-19-24-15-29(41-47-24)31-27(37)7-8-28(38)32(31)36/h2-3,6-9,14-16,23,25,29,39,41,44H,4-5,10-13,17-19H2,1H3/t25-,29?,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in buffer |
Bioorg Med Chem Lett 20: 6291-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.087
BindingDB Entry DOI: 10.7270/Q2H70G2C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data