| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor alpha |
|---|
| Ligand | BDBM50109546 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_153400 (CHEMBL763853) |
|---|
| EC50 | 960±n/a nM |
|---|
| Citation |  Sauerberg, P; Bury, PS; Mogensen, JP; Deussen, HJ; Pettersson, I; Fleckner, J; Nehlin, J; Frederiksen, KS; Albrektsen, T; Din, N; Svensson, LA; Ynddal, L; Wulff, EM; Jeppesen, L Large dimeric ligands with favorable pharmacokinetic properties and peroxisome proliferator-activated receptor agonist activity in vitro and in vivo. J Med Chem46:4883-94 (2003) [PubMed] Article Sauerberg, P; Bury, PS; Mogensen, JP; Deussen, HJ; Pettersson, I; Fleckner, J; Nehlin, J; Frederiksen, KS; Albrektsen, T; Din, N; Svensson, LA; Ynddal, L; Wulff, EM; Jeppesen, L Large dimeric ligands with favorable pharmacokinetic properties and peroxisome proliferator-activated receptor agonist activity in vitro and in vivo. J Med Chem46:4883-94 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor alpha |
|---|
| Name: | Peroxisome proliferator-activated receptor alpha |
|---|
| Synonyms: | NR1C1 | Nuclear receptor subfamily 1 group C member 1 | PPAR | PPAR alpha/gamma | PPAR-alpha | PPARA | PPARA_HUMAN | Peroxisome Proliferator-Activated Receptor alpha | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor alpha (PPAR alpha) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 52222.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q07869 |
|---|
| Residue: | 468 |
|---|
| Sequence: | MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSC
PGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACE
GCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSE
KAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFV
IHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANL
DLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFD
FAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDI
FLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY
|
|
|
|---|
| BDBM50109546 |
|---|
| n/a |
|---|
| Name | BDBM50109546 |
|---|
| Synonyms: | 5-[(3aS,4R,5R,6aS)-5-Hydroxy-4-((S)-3-hydroxy-oct-1-enyl)-hexahydro-pentalen-(2Z)-ylidene]-pentanoic acid | 5-[5-Hydroxy-4-(3-hydroxy-oct-1-enyl)-hexahydro-pentalen-2-ylidene]-pentanoic acidCarbacyclin | CARBACYCLIN | CHEMBL148319 | PGI2 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H34O4 |
|---|
| Mol. Mass. | 350.4923 |
|---|
| SMILES | CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)C[C@@H]2C\C(C[C@H]12)=C\CCCC(O)=O |
|---|
| Structure | 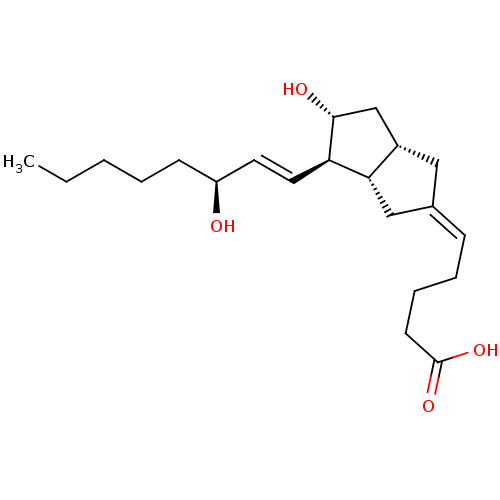
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sauerberg, P; Bury, PS; Mogensen, JP; Deussen, HJ; Pettersson, I; Fleckner, J; Nehlin, J; Frederiksen, KS; Albrektsen, T; Din, N; Svensson, LA; Ynddal, L; Wulff, EM; Jeppesen, L Large dimeric ligands with favorable pharmacokinetic properties and peroxisome proliferator-activated receptor agonist activity in vitro and in vivo. J Med Chem46:4883-94 (2003) [PubMed] Article
Sauerberg, P; Bury, PS; Mogensen, JP; Deussen, HJ; Pettersson, I; Fleckner, J; Nehlin, J; Frederiksen, KS; Albrektsen, T; Din, N; Svensson, LA; Ynddal, L; Wulff, EM; Jeppesen, L Large dimeric ligands with favorable pharmacokinetic properties and peroxisome proliferator-activated receptor agonist activity in vitro and in vivo. J Med Chem46:4883-94 (2003) [PubMed] Article