| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone deacetylase 2b |
|---|
| Ligand | BDBM50140882 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_87886 (CHEMBL698804) |
|---|
| IC50 | 50±n/a nM |
|---|
| Citation |  Ragno, R; Mai, A; Massa, S; Cerbara, I; Valente, S; Bottoni, P; Scatena, R; Jesacher, F; Loidl, P; Brosch, G 3-(4-Aroyl-1-methyl-1H-pyrrol-2-yl)-N-hydroxy-2-propenamides as a new class of synthetic histone deacetylase inhibitors. 3. Discovery of novel lead compounds through structure-based drug design and docking studies. J Med Chem47:1351-9 (2004) [PubMed] Article Ragno, R; Mai, A; Massa, S; Cerbara, I; Valente, S; Bottoni, P; Scatena, R; Jesacher, F; Loidl, P; Brosch, G 3-(4-Aroyl-1-methyl-1H-pyrrol-2-yl)-N-hydroxy-2-propenamides as a new class of synthetic histone deacetylase inhibitors. 3. Discovery of novel lead compounds through structure-based drug design and docking studies. J Med Chem47:1351-9 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone deacetylase 2b |
|---|
| Name: | Histone deacetylase 2b |
|---|
| Synonyms: | Histone deacetylase HD2 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 30785.07 |
|---|
| Organism: | Zea mays |
|---|
| Description: | ChEMBL_87549 |
|---|
| Residue: | 286 |
|---|
| Sequence: | MEVGGQEVKPGATVSCKVGDGLVIHLSQAALGESKKASENAILSVNIDDKKLVLGTLSVE
KHPQISCDLVFDKDFELPHNSKTRSVFFRGYKSPVPLFESNSGEDSSDEELKTDQIPLQN
NEIKISAAKVPAKDDDDDVFIILAMMMMIYSSDDDDDDFTTSDSDNEMSEEDDSSDEDEM
SEEDDSSDEDEMSGGADPSDDSSDESGSEHTSAPKKTDVVVGKKRAIKAEAPYGKKAKSE
QSSQKTGDKASTSHPAKQSIKTPADKSRKTPTADKKSPKSGSHGCK
|
|
|
|---|
| BDBM50140882 |
|---|
| n/a |
|---|
| Name | BDBM50140882 |
|---|
| Synonyms: | (E)-3-(5-Benzoyl-1-methyl-1H-pyrrol-3-yl)-N-hydroxy-acrylamide | 3-(5-benzoyl-1-methyl-1H-pyrrol-3-yl)-N-hydroxyacrylamide | CHEMBL29814 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H14N2O3 |
|---|
| Mol. Mass. | 270.2833 |
|---|
| SMILES | Cn1cc(\C=C\C(=O)NO)cc1C(=O)c1ccccc1 |
|---|
| Structure | 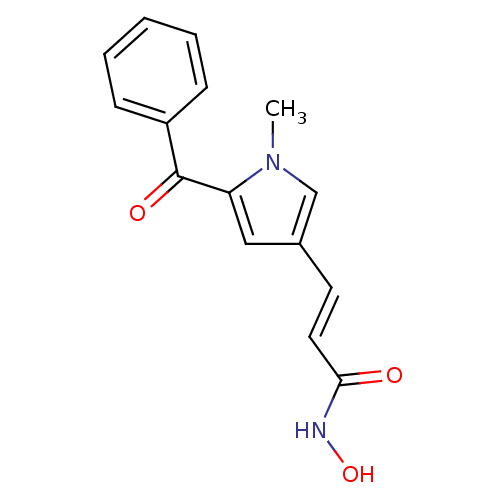
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ragno, R; Mai, A; Massa, S; Cerbara, I; Valente, S; Bottoni, P; Scatena, R; Jesacher, F; Loidl, P; Brosch, G 3-(4-Aroyl-1-methyl-1H-pyrrol-2-yl)-N-hydroxy-2-propenamides as a new class of synthetic histone deacetylase inhibitors. 3. Discovery of novel lead compounds through structure-based drug design and docking studies. J Med Chem47:1351-9 (2004) [PubMed] Article
Ragno, R; Mai, A; Massa, S; Cerbara, I; Valente, S; Bottoni, P; Scatena, R; Jesacher, F; Loidl, P; Brosch, G 3-(4-Aroyl-1-methyl-1H-pyrrol-2-yl)-N-hydroxy-2-propenamides as a new class of synthetic histone deacetylase inhibitors. 3. Discovery of novel lead compounds through structure-based drug design and docking studies. J Med Chem47:1351-9 (2004) [PubMed] Article