

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50026872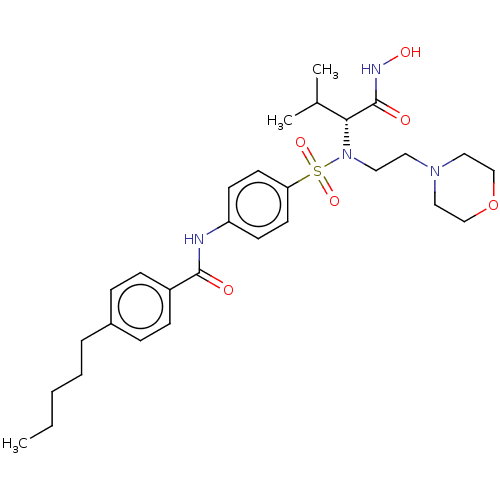 (CHEMBL1233506 | SC-74020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured after 3... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50366175 (CHEMBL1957606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of MMP9 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured af... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50366175 (CHEMBL1957606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured after 3... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50366203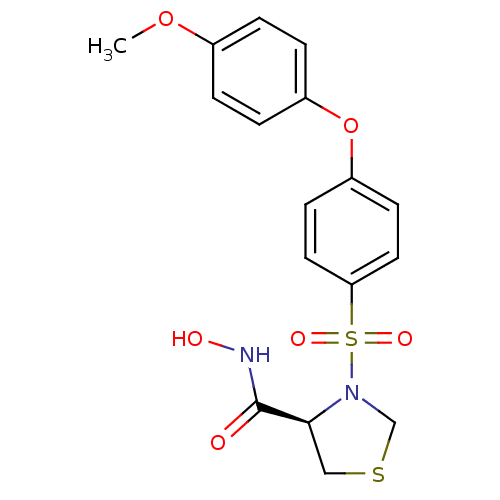 (CHEMBL1957601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of MMP9 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured af... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of mouse HDAC1 | J Med Chem 47: 1351-9 (2004) Article DOI: 10.1021/jm031036f BindingDB Entry DOI: 10.7270/Q2X63MCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50366175 (CHEMBL1957606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of MMP8 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured af... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50366205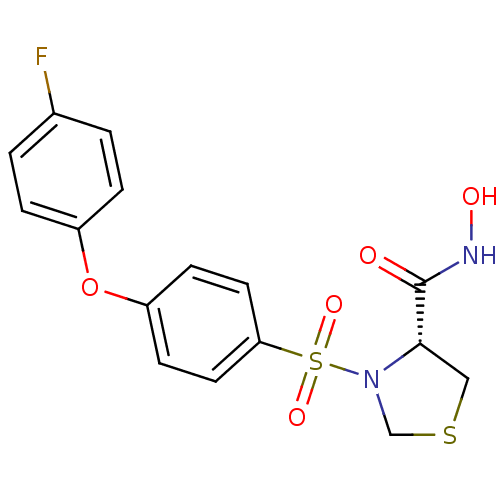 (CHEMBL1957603) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of MMP9 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured af... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50366175 (CHEMBL1957606) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of MMP14 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured a... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of maize Histone deacetylase 2 | J Med Chem 47: 1351-9 (2004) Article DOI: 10.1021/jm031036f BindingDB Entry DOI: 10.7270/Q2X63MCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of maize histone deacetylase 2 | J Med Chem 47: 1351-9 (2004) Article DOI: 10.1021/jm031036f BindingDB Entry DOI: 10.7270/Q2X63MCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50366173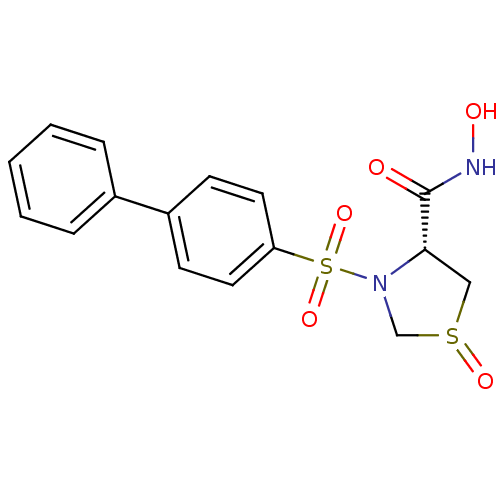 (CHEMBL1957604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured after 3... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50366203 (CHEMBL1957601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured after 3... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50134805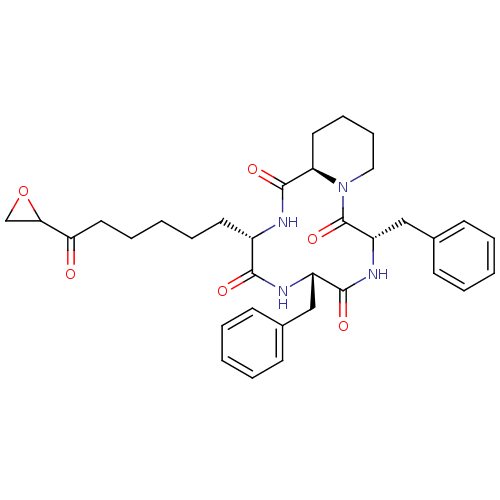 (6,9-Dibenzyl-12-(6-oxiranyl-6-oxo-hexyl)-decahydro...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50366205 (CHEMBL1957603) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured after 3... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50366204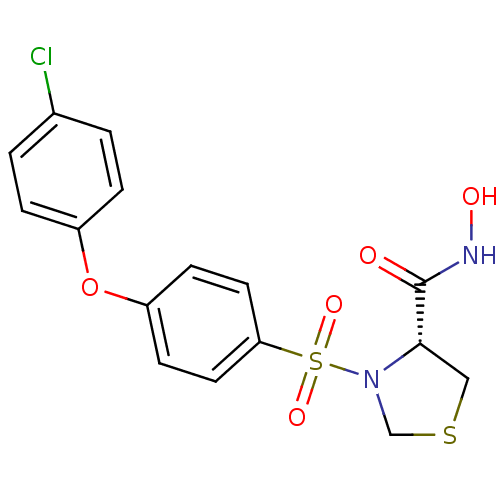 (CHEMBL1957602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured after 3... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50366204 (CHEMBL1957602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of MMP9 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured af... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50366175 (CHEMBL1957606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of MMP12 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured a... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139952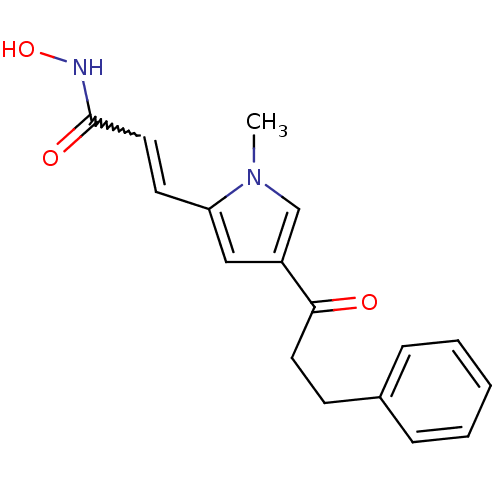 (CHEMBL268286 | N-Hydroxy-3-[1-methyl-4-(3-phenyl-p...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139952 (CHEMBL268286 | N-Hydroxy-3-[1-methyl-4-(3-phenyl-p...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50140882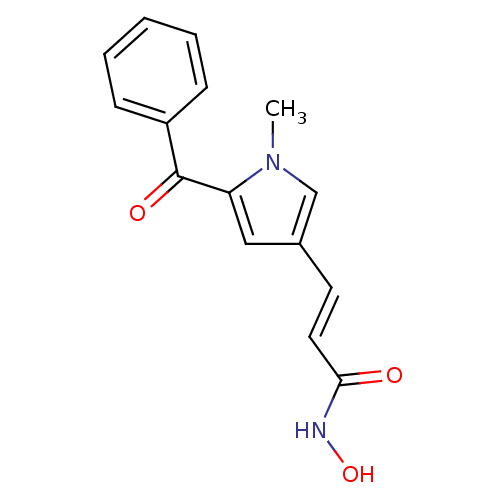 ((E)-3-(5-Benzoyl-1-methyl-1H-pyrrol-3-yl)-N-hydrox...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of maize histone deacetylase 2 | J Med Chem 47: 1351-9 (2004) Article DOI: 10.1021/jm031036f BindingDB Entry DOI: 10.7270/Q2X63MCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of maize histone deacetylase 2 | J Med Chem 47: 1351-9 (2004) Article DOI: 10.1021/jm031036f BindingDB Entry DOI: 10.7270/Q2X63MCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50140882 ((E)-3-(5-Benzoyl-1-methyl-1H-pyrrol-3-yl)-N-hydrox...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of maize Histone deacetylase 2 | J Med Chem 47: 1351-9 (2004) Article DOI: 10.1021/jm031036f BindingDB Entry DOI: 10.7270/Q2X63MCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of maize Histone deacetylase 2 | J Med Chem 47: 1351-9 (2004) Article DOI: 10.1021/jm031036f BindingDB Entry DOI: 10.7270/Q2X63MCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50366174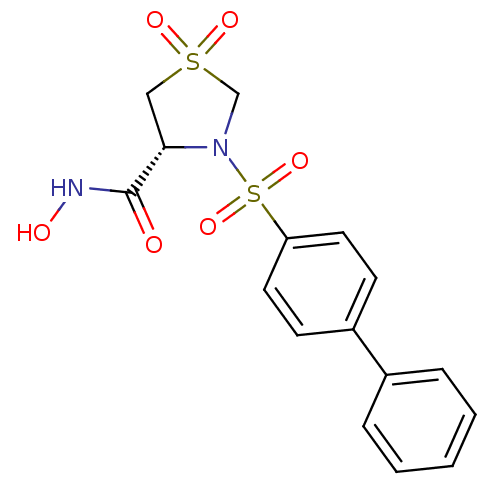 (CHEMBL1957605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured after 3... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139949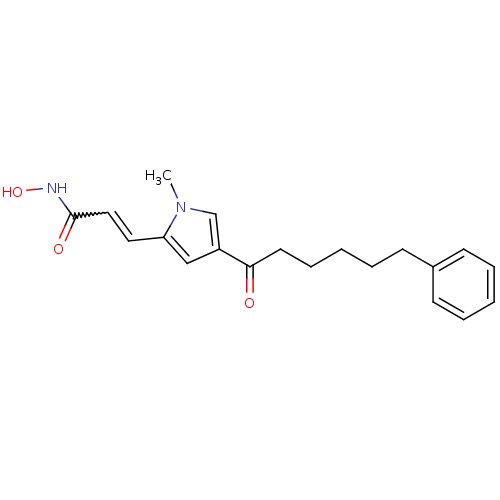 (CHEMBL13200 | N-Hydroxy-3-[1-methyl-4-(6-phenyl-he...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139949 (CHEMBL13200 | N-Hydroxy-3-[1-methyl-4-(6-phenyl-he...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibitory concentration against histone deacetylase HD2 enzyme. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50135752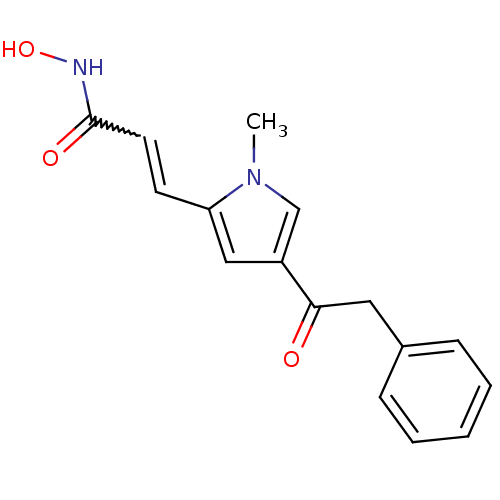 ((2E)-N-hydroxy-3-[1-methyl-4-(phenylacetyl)-1H-pyr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibitory concentration against histone deacetylase HD2 enzyme. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139935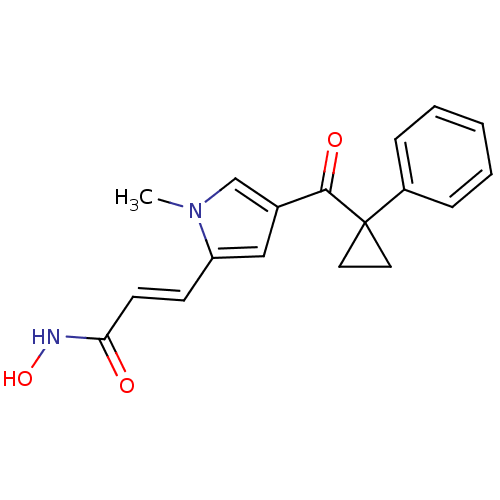 (CHEMBL12927 | N-Hydroxy-3-[1-methyl-4-(1-phenyl-cy...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50366174 (CHEMBL1957605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of MMP8 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured af... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50135752 ((2E)-N-hydroxy-3-[1-methyl-4-(phenylacetyl)-1H-pyr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibitory concentration against histone deacetylase HD2 enzyme. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139935 (CHEMBL12927 | N-Hydroxy-3-[1-methyl-4-(1-phenyl-cy...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139942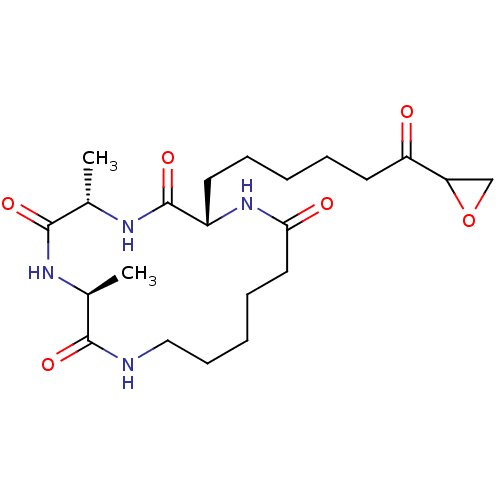 ((5S,8S,11S)-5,8-Dimethyl-11-(6-oxiranyl-6-oxo-hexy...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139937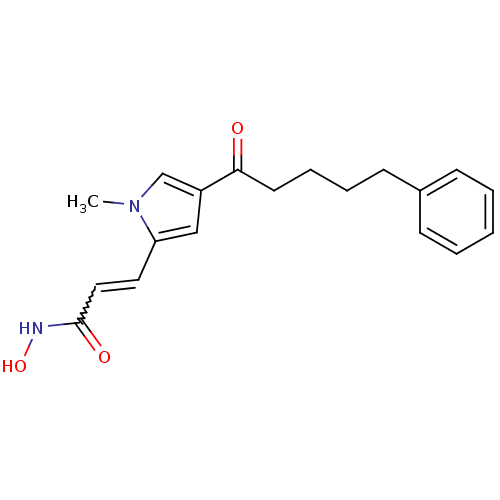 (CHEMBL274906 | N-Hydroxy-3-[1-methyl-4-(5-phenyl-p...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139937 (CHEMBL274906 | N-Hydroxy-3-[1-methyl-4-(5-phenyl-p...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibitory concentration against histone deacetylase HD2 enzyme. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma "La Sapienza" Curated by ChEMBL | Assay Description Inhibition of mouse HDAC1 | J Med Chem 47: 1351-9 (2004) Article DOI: 10.1021/jm031036f BindingDB Entry DOI: 10.7270/Q2X63MCK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139945 (CHEMBL273731 | N-Hydroxy-3-[1-methyl-4-(naphthalen...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139945 (CHEMBL273731 | N-Hydroxy-3-[1-methyl-4-(naphthalen...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139947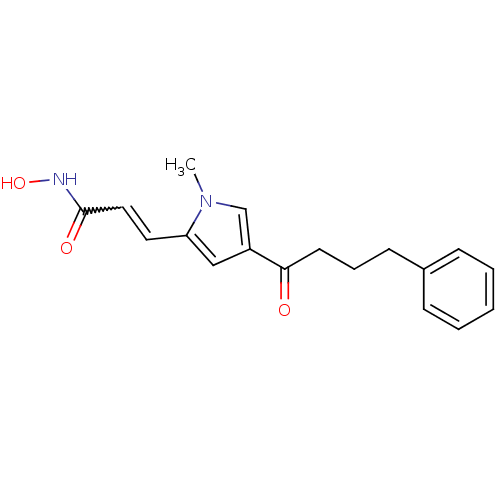 (CHEMBL265077 | N-Hydroxy-3-[1-methyl-4-(4-phenyl-b...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibitory concentration against histone deacetylase HD2 enzyme. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139947 (CHEMBL265077 | N-Hydroxy-3-[1-methyl-4-(4-phenyl-b...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139944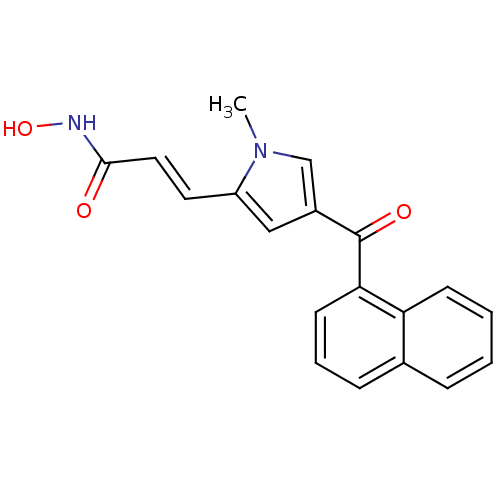 (CHEMBL12890 | N-Hydroxy-3-[1-methyl-4-(naphthalene...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139944 (CHEMBL12890 | N-Hydroxy-3-[1-methyl-4-(naphthalene...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibitory concentration against histone deacetylase HD2 enzyme. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50366173 (CHEMBL1957604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of MMP14 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured a... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50366204 (CHEMBL1957602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of MMP3 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured af... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139930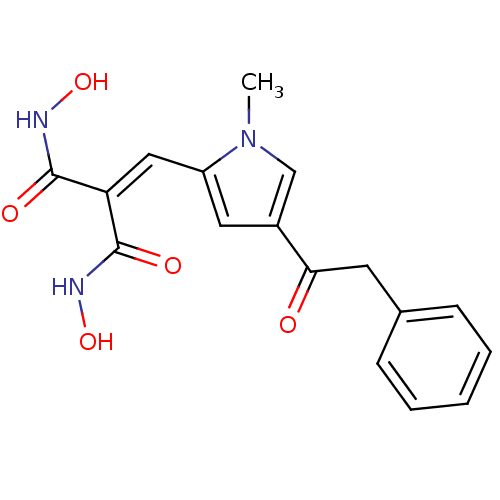 (CHEMBL269230 | N,N'-Dihydroxy-2-(1-methyl-4-phenyl...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibitory concentration against histone deacetylase HD2 enzyme. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50366200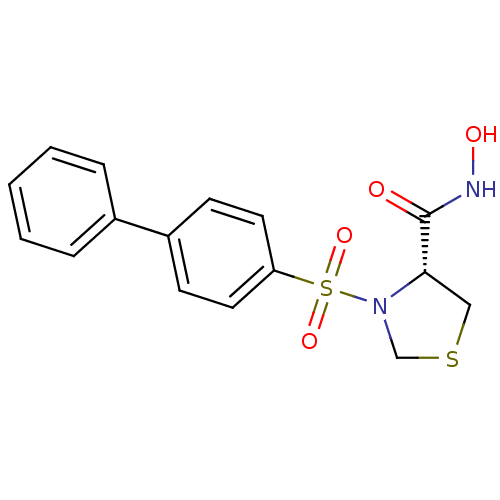 (CHEMBL1957598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl) Curated by ChEMBL | Assay Description Inhibition of full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured after 3... | Bioorg Med Chem 20: 2323-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.010 BindingDB Entry DOI: 10.7270/Q2N29XD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139930 (CHEMBL269230 | N,N'-Dihydroxy-2-(1-methyl-4-phenyl...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Concentration required for inhibition of histone deacetylase HD2 in vitro. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139951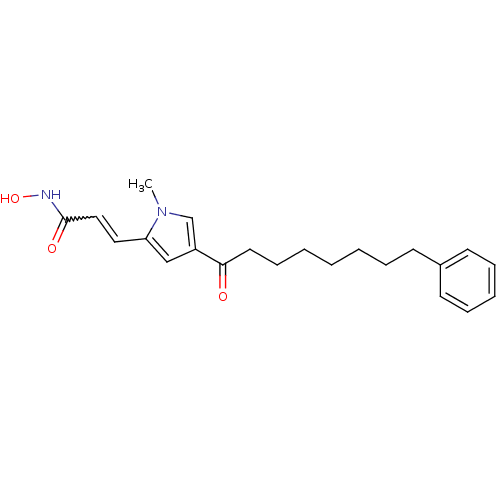 (CHEMBL273454 | N-Hydroxy-3-[1-methyl-4-(8-phenyl-o...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibitory concentration against histone deacetylase HD2 enzyme. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50139951 (CHEMBL273454 | N-Hydroxy-3-[1-methyl-4-(8-phenyl-o...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibitory concentration against histone deacetylase HD2 enzyme. | J Med Chem 47: 1098-109 (2004) Checked by Author Article DOI: 10.1021/jm030990+ BindingDB Entry DOI: 10.7270/Q24X5773 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 141 total ) | Next | Last >> |