| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peptidyl-prolyl cis-trans isomerase FKBP5 |
|---|
| Ligand | BDBM50575667 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2125785 (CHEMBL4835130) |
|---|
| Kd | 6.9±n/a nM |
|---|
| Citation |  Bauder, M; Meyners, C; Purder, PL; Merz, S; Sugiarto, WO; Voll, AM; Heymann, T; Hausch, F Structure-Based Design of High-Affinity Macrocyclic FKBP51 Inhibitors. J Med Chem64:3320-3349 (2021) [PubMed] Article Bauder, M; Meyners, C; Purder, PL; Merz, S; Sugiarto, WO; Voll, AM; Heymann, T; Hausch, F Structure-Based Design of High-Affinity Macrocyclic FKBP51 Inhibitors. J Med Chem64:3320-3349 (2021) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peptidyl-prolyl cis-trans isomerase FKBP5 |
|---|
| Name: | Peptidyl-prolyl cis-trans isomerase FKBP5 |
|---|
| Synonyms: | 51 kDa FK506-binding protein | 51 kDa FKBP | 54 kDa progesterone receptor-associated immunophilin | AIG6 | Androgen-regulated protein 6 | FF1 antigen | FK506-binding protein 5 | FKBP-5 | FKBP-51 | FKBP5 | FKBP51 | FKBP54 | FKBP5_HUMAN | HSP90-binding immunophilin | PPIase FKBP5 | Rotamase | p54 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 51207.06 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_952910 |
|---|
| Residue: | 457 |
|---|
| Sequence: | MTTDEGAKNNEESPTATVAEQGEDITSKKDRGVLKIVKRVGNGEETPMIGDKVYVHYKGK
LSNGKKFDSSHDRNEPFVFSLGKGQVIKAWDIGVATMKKGEICHLLCKPEYAYGSAGSLP
KIPSNATLFFEIELLDFKGEDLFEDGGIIRRTKRKGEGYSNPNEGATVEIHLEGRCGGRM
FDCRDVAFTVGEGEDHDIPIGIDKALEKMQREEQCILYLGPRYGFGEAGKPKFGIEPNAE
LIYEVTLKSFEKAKESWEMDTKEKLEQAAIVKEKGTVYFKGGKYMQAVIQYGKIVSWLEM
EYGLSEKESKASESFLLAAFLNLAMCYLKLREYTKAVECCDKALGLDSANEKGLYRRGEA
QLLMNEFESAKGDFEKVLEVNPQNKAARLQISMCQKKAKEHNERDRRIYANMFKKFAEQD
AKEEANKAMGKKTSEGVTNEKGTDSQAMEEEKPEGHV
|
|
|
|---|
| BDBM50575667 |
|---|
| n/a |
|---|
| Name | BDBM50575667 |
|---|
| Synonyms: | CHEMBL4871144 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C43H55NO11 |
|---|
| Mol. Mass. | 761.8969 |
|---|
| SMILES | COc1ccc(CC[C@H]2OC(=O)[C@@H]3CCCCN3C(=O)[C@@H](C3CCCCC3)c3cc(OC)c(OC)c(OC[C@@H](O)[C@H](O)COc4cccc2c4)c3)cc1OC |r| |
|---|
| Structure | 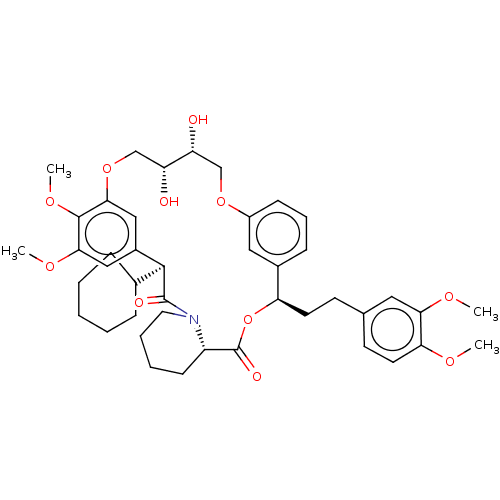
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bauder, M; Meyners, C; Purder, PL; Merz, S; Sugiarto, WO; Voll, AM; Heymann, T; Hausch, F Structure-Based Design of High-Affinity Macrocyclic FKBP51 Inhibitors. J Med Chem64:3320-3349 (2021) [PubMed] Article
Bauder, M; Meyners, C; Purder, PL; Merz, S; Sugiarto, WO; Voll, AM; Heymann, T; Hausch, F Structure-Based Design of High-Affinity Macrocyclic FKBP51 Inhibitors. J Med Chem64:3320-3349 (2021) [PubMed] Article