

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263407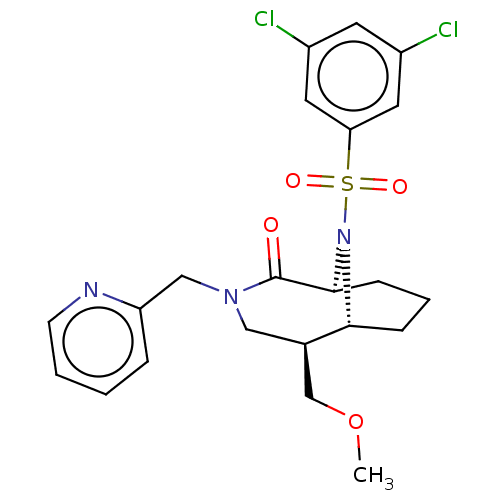 (CHEMBL4090599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50263407 (CHEMBL4090599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM36609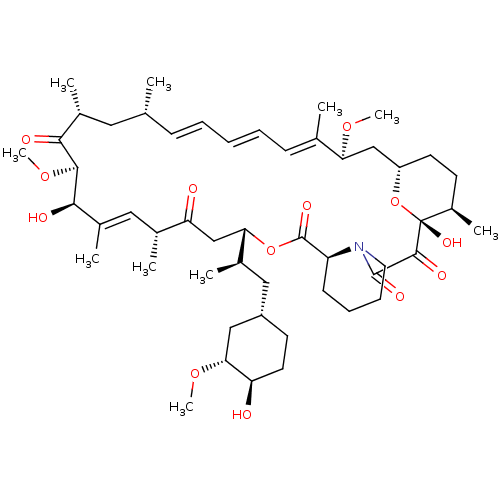 (Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to human FKBP51 by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125333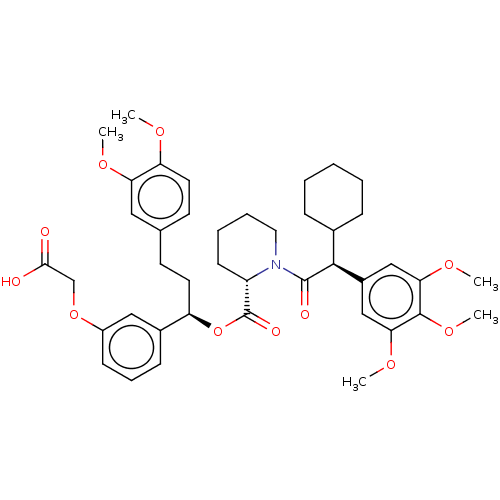 (CHEMBL3623612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP51 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 59: 2410-22 (2016) Article DOI: 10.1021/acs.jmedchem.5b01355 BindingDB Entry DOI: 10.7270/Q2BZ67Z3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125333 (CHEMBL3623612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of iFit-FL from FKBP51 (unknown origin) by fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125330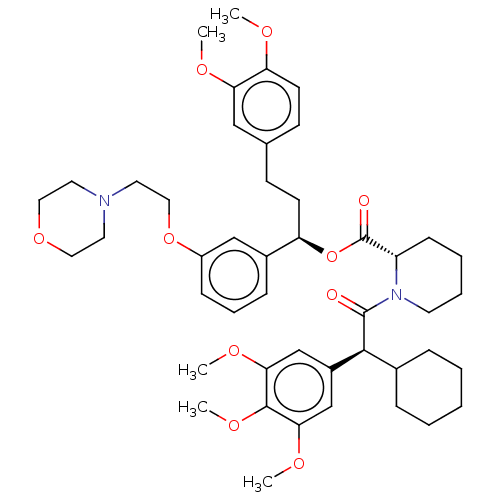 (CHEMBL3623630) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP51 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 59: 2410-22 (2016) Article DOI: 10.1021/acs.jmedchem.5b01355 BindingDB Entry DOI: 10.7270/Q2BZ67Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125330 (CHEMBL3623630) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of iFit-FL from FKBP51 (unknown origin) by fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of fluorescein labeled cyclosporin binding to Cyp40 by flourescence polarization competition assay | ACS Med Chem Lett 1: 536-539 (2010) Article DOI: 10.1021/ml1001272 BindingDB Entry DOI: 10.7270/Q26110M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263435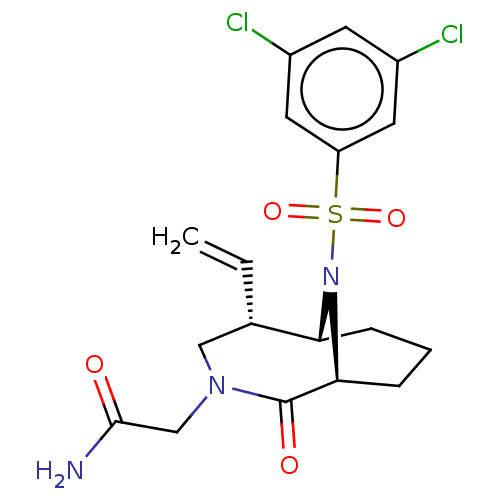 (CHEMBL4075704) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263422 (CHEMBL4072643) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263421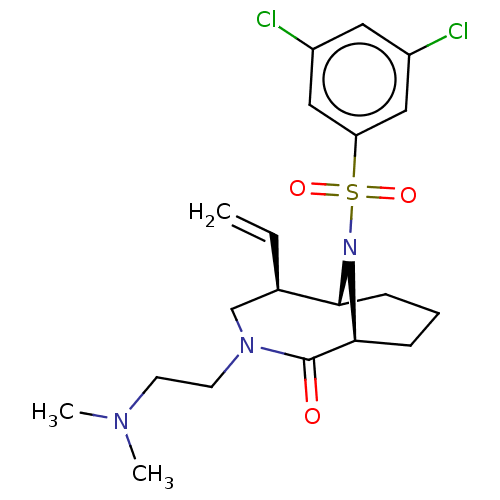 (CHEMBL4101268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263420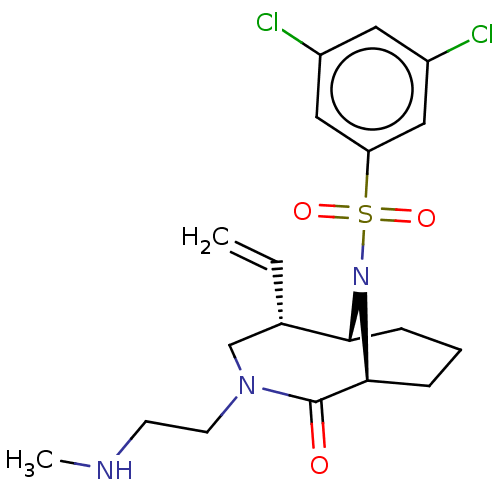 (CHEMBL4102121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263416 (CHEMBL4067970) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263408 (CHEMBL4063858) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50339005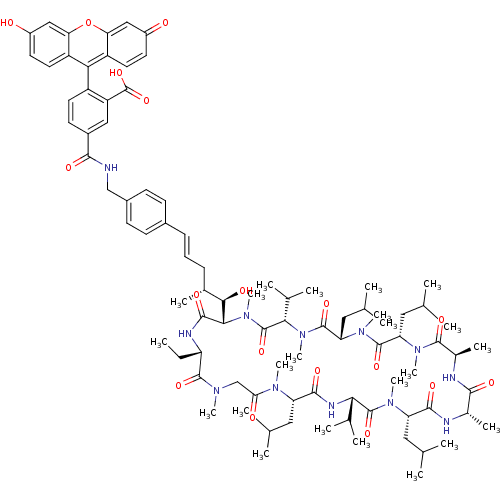 (5-(4-((4R,5R,E)-5-((2S,5S,11S,14S,17S,20S,23R,26S,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cyclophilin 40 PPIase activity | ACS Med Chem Lett 1: 536-539 (2010) Article DOI: 10.1021/ml1001272 BindingDB Entry DOI: 10.7270/Q26110M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263417 (CHEMBL4068304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50263407 (CHEMBL4090599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263424 (CHEMBL4077424) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263437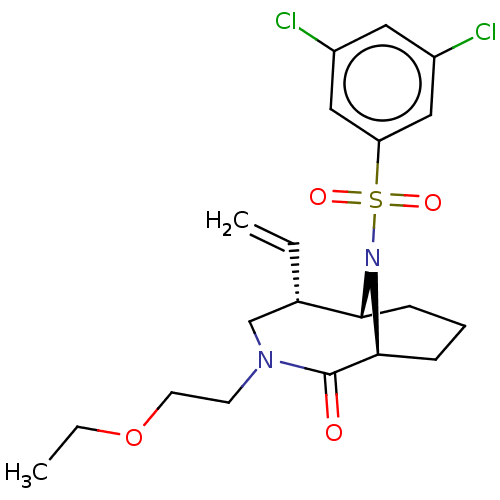 (CHEMBL4073944) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50263407 (CHEMBL4090599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cyclophilin 40 PPIase activity | ACS Med Chem Lett 1: 536-539 (2010) Article DOI: 10.1021/ml1001272 BindingDB Entry DOI: 10.7270/Q26110M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50162500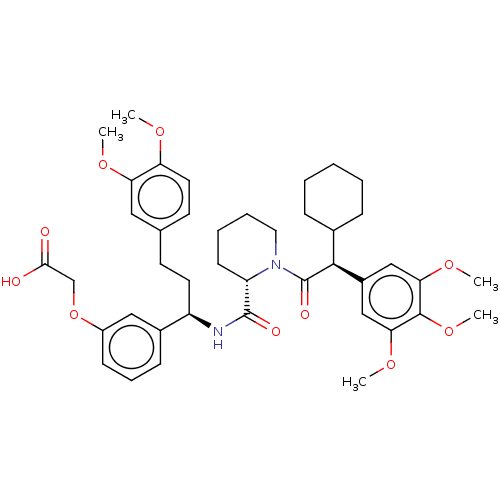 (CHEMBL3792975) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP51 FK506-binding domain (1 to 140 amino acids) (unknown origin) incubated for 30 mins using fluorescein-conjugated 2-(5-((2-(... | J Med Chem 59: 2410-22 (2016) Article DOI: 10.1021/acs.jmedchem.5b01355 BindingDB Entry DOI: 10.7270/Q2BZ67Z3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263423 (CHEMBL4077610) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263394 (CHEMBL4069155) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263436 (CHEMBL4075784) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263439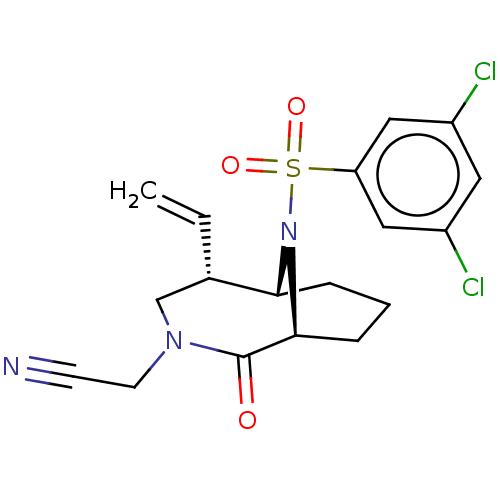 (CHEMBL4076387) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263409 (CHEMBL4086695) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP2 (Homo sapiens) | BDBM50263407 (CHEMBL4090599) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50030448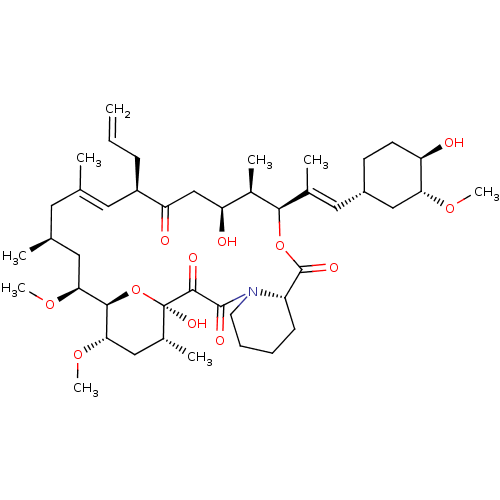 (8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP51 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 59: 2410-22 (2016) Article DOI: 10.1021/acs.jmedchem.5b01355 BindingDB Entry DOI: 10.7270/Q2BZ67Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50432752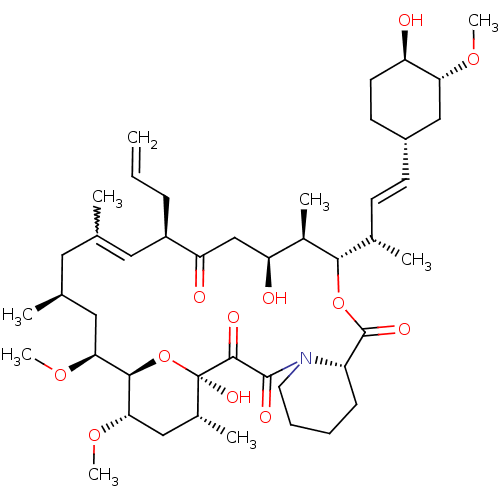 (FK-506) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to human FKBP51 by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50125333 (CHEMBL3623612) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP12 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50339005 (5-(4-((4R,5R,E)-5-((2S,5S,11S,14S,17S,20S,23R,26S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cyclophilin 18 PPIase activity | ACS Med Chem Lett 1: 536-539 (2010) Article DOI: 10.1021/ml1001272 BindingDB Entry DOI: 10.7270/Q26110M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125332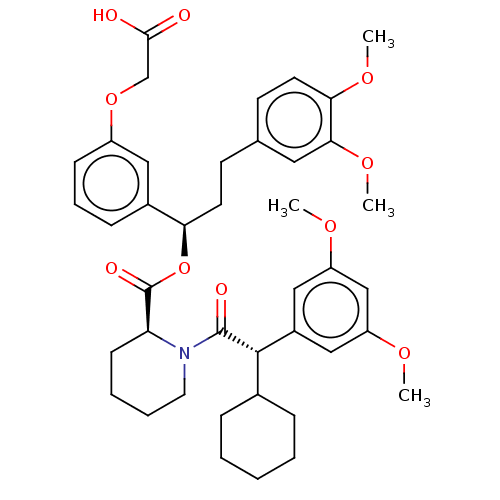 (CHEMBL3623613) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of iFit-FL from FKBP51 (unknown origin) by fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50162684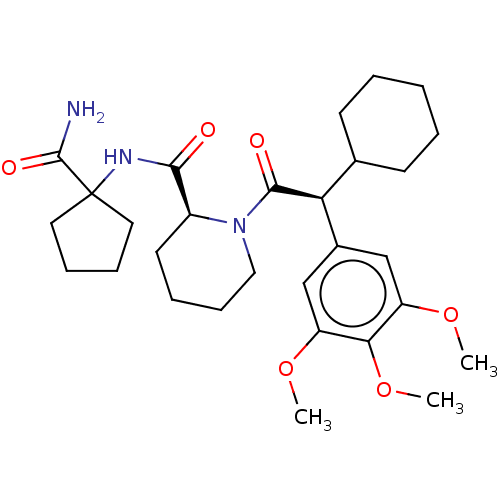 (CHEMBL3793603) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP51 FK506-binding domain (1 to 140 amino acids) (unknown origin) incubated for 30 mins using fluorescein-conjugated 2-(5-((2-(... | J Med Chem 59: 2410-22 (2016) Article DOI: 10.1021/acs.jmedchem.5b01355 BindingDB Entry DOI: 10.7270/Q2BZ67Z3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263414 (CHEMBL4104603) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50031240 (CHEMBL3357043) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human histamine H1 receptor expressed in Sf9 cells by scintillation counting method | J Med Chem 57: 9473-9 (2014) Article DOI: 10.1021/jm501086v BindingDB Entry DOI: 10.7270/Q22F7Q1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50031115 (CHEMBL3357041) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human histamine H1 receptor expressed in Sf9 cells by scintillation counting method | J Med Chem 57: 9473-9 (2014) Article DOI: 10.1021/jm501086v BindingDB Entry DOI: 10.7270/Q22F7Q1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50125331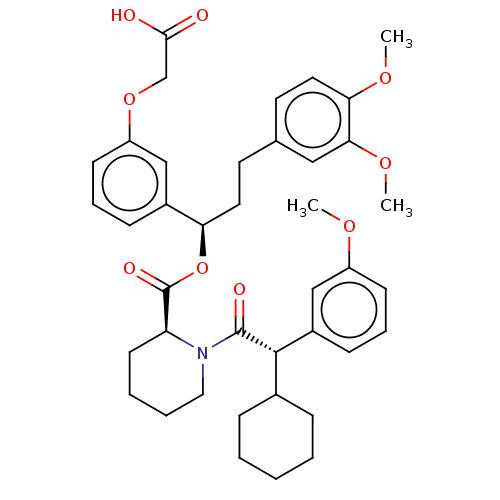 (CHEMBL3623614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of iFit-FL from FKBP51 (unknown origin) by fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50007465 (1-[4-(8-Chloro-5,6-dihydro-benzo[5,6]cyclohepta[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human histamine H1 receptor expressed in Sf9 cells by scintillation counting method | J Med Chem 57: 9473-9 (2014) Article DOI: 10.1021/jm501086v BindingDB Entry DOI: 10.7270/Q22F7Q1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50388298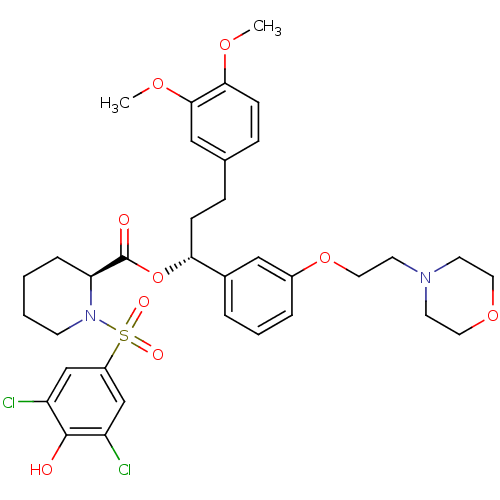 (CHEMBL2058794) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to human FKBP51 by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cyclophilin 18 PPIase activity | ACS Med Chem Lett 1: 536-539 (2010) Article DOI: 10.1021/ml1001272 BindingDB Entry DOI: 10.7270/Q26110M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50519836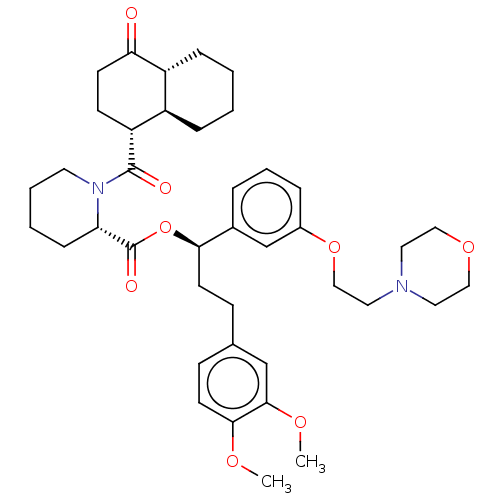 (CHEMBL4588417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged/C-terminal FLAG tagged FKBP51 expressed in Escherichia coli BL21 (DE3) cells incubated for 30 m... | J Med Chem 63: 231-240 (2020) Article DOI: 10.1021/acs.jmedchem.9b01157 BindingDB Entry DOI: 10.7270/Q2Q52T10 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of fluorescein labeled cyclosporin binding to Cyp18 by flourescence polarization competition assay | ACS Med Chem Lett 1: 536-539 (2010) Article DOI: 10.1021/ml1001272 BindingDB Entry DOI: 10.7270/Q26110M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50263415 (CHEMBL4072633) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50519830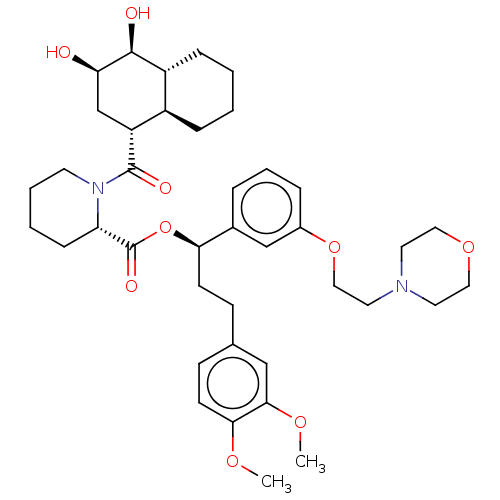 (CHEMBL4463025) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Inhibition of human full length N-terminal His-tagged/C-terminal FLAG tagged FKBP51 expressed in Escherichia coli BL21 (DE3) cells incubated for 30 m... | J Med Chem 63: 231-240 (2020) Article DOI: 10.1021/acs.jmedchem.9b01157 BindingDB Entry DOI: 10.7270/Q2Q52T10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50031122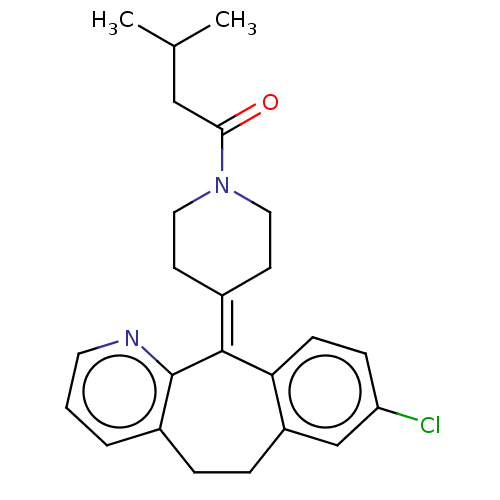 (CHEMBL3357042) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human histamine H1 receptor expressed in Sf9 cells by scintillation counting method | J Med Chem 57: 9473-9 (2014) Article DOI: 10.1021/jm501086v BindingDB Entry DOI: 10.7270/Q22F7Q1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP5 (Homo sapiens (Human)) | BDBM50432732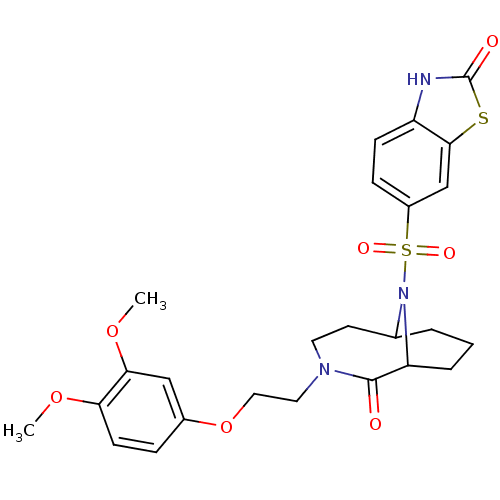 (CHEMBL2348594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to human FKBP51 by competitive fluorescence polarization assay | J Med Chem 56: 3922-35 (2013) Article DOI: 10.1021/jm400087k BindingDB Entry DOI: 10.7270/Q2QZ2CC1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50125326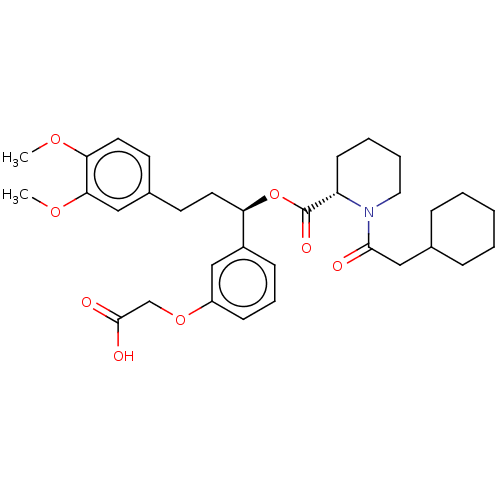 (CHEMBL3623618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Binding affinity to FKBP12 (unknown origin) by competitive fluorescence polarization assay | J Med Chem 58: 7796-806 (2015) Article DOI: 10.1021/acs.jmedchem.5b00785 BindingDB Entry DOI: 10.7270/Q25Q4XWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22876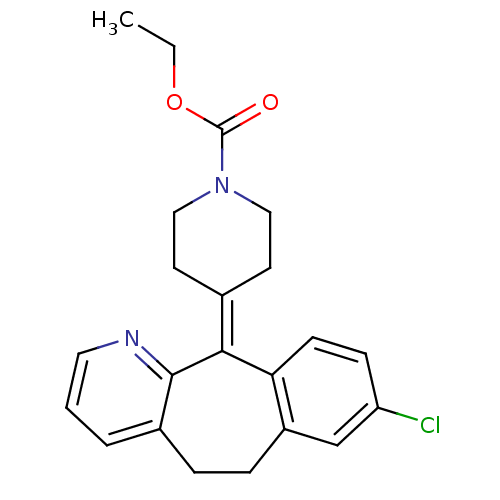 (CHEMBL998 | Claritin | Loratadine | Sch 29851 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Institute of Psychiatry Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from human histamine H1 receptor expressed in Sf9 cells by scintillation counting method | J Med Chem 57: 9473-9 (2014) Article DOI: 10.1021/jm501086v BindingDB Entry DOI: 10.7270/Q22F7Q1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase Mip (Chlamydia pneumoniae) | BDBM50263406 (CHEMBL4089167) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 315 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Translational Research in Psychiatry , Max Planck Institute of Psychiatry , 80804 Munich , Germany. Curated by ChEMBL | Assay Description Displacement of 5-(3-(4-(((5S,6S)-10-(3,5-dichlorophenylsulfonyl)-2-oxo-5-vinyl-3,10-diazabicyclo[4.3.1]decan-3-yl)methyl)-1H-1,2,3-triazol-1-yl)prop... | J Med Chem 61: 3660-3673 (2018) Article DOI: 10.1021/acs.jmedchem.8b00137 BindingDB Entry DOI: 10.7270/Q2833VGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 598 total ) | Next | Last >> |