

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Vascular endothelial growth factor receptor 2 | ||
| Ligand | BDBM8797 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEBML_214087 | ||
| IC50 | >50000±n/a nM | ||
| Citation |  Borhani, DW; Calderwood, DJ; Friedman, MM; Hirst, GC; Li, B; Leung, AK; McRae, B; Ratnofsky, S; Ritter, K; Waegell, W A-420983: a potent, orally active inhibitor of lck with efficacy in a model of transplant rejection. Bioorg Med Chem Lett14:2613-6 (2004) [PubMed] Article Borhani, DW; Calderwood, DJ; Friedman, MM; Hirst, GC; Li, B; Leung, AK; McRae, B; Ratnofsky, S; Ritter, K; Waegell, W A-420983: a potent, orally active inhibitor of lck with efficacy in a model of transplant rejection. Bioorg Med Chem Lett14:2613-6 (2004) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Vascular endothelial growth factor receptor 2 | |||
| Name: | Vascular endothelial growth factor receptor 2 | ||
| Synonyms: | CD_antigen=CD309 | FLK1 | Fetal liver kinase 1 (FLK-1) | Flk-1/KDR | KDR | Kinase Insert Domain Receptor | Protein-tyrosine kinase receptor Flk-1 | VEGFR kinase (KDR) | VEGFR-2 | VEGFR-2 (KDR) | VEGFR2 | VGFR2_HUMAN | Vascular Endothelial Growth Factor Receptor Kinase 2 | Vascular endothelial growth factor receptor (VEGFR-2) | Vascular endothelial growth factor receptor 2 (KDR) | Vascular endothelial growth factor receptor 2 (VEGFR-2) | Vascular endothelial growth factor receptor 2 (VEGFR2) | Vascular endothelial growth factor receptor 2 precursor (VEGFR-2) | Vascular endothelial growth factor receptor-2 (VEGFR-2) | ||
| Type: | Receptor Tyrosine Kinase | ||
| Mol. Mass.: | 151510.97 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P35968 | ||
| Residue: | 1356 | ||
| Sequence: |
| ||
| BDBM8797 | |||
| n/a | |||
| Name | BDBM8797 | ||
| Synonyms: | CHEMBL297363 | N-(4-{4-amino-1-[4-(4-methylpiperazin-1-yl)cyclohexyl]-1H-pyrazolo[3,4-d]pyrimidin-3-yl}-2-methoxyphenyl)benzamide | Pyrazolo[3,4-d]pyrimidine 5 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C30H36N8O2 | ||
| Mol. Mass. | 540.6592 | ||
| SMILES | COc1cc(ccc1NC(=O)c1ccccc1)-c1nn([C@H]2CC[C@@H](CC2)N2CCN(C)CC2)c2ncnc(N)c12 |r,wU:20.21,wD:23.28,(.85,5.16,;-.29,6.2,;-1.75,5.73,;-2.08,4.23,;-3.55,3.76,;-4.69,4.8,;-4.36,6.3,;-2.89,6.77,;-2.56,8.27,;-1.1,8.74,;.04,7.7,;-.77,10.25,;-1.86,11.33,;-1.46,12.82,;.02,13.22,;1.12,12.14,;.72,10.65,;-4.03,2.3,;-3.12,1.05,;-4.03,-.2,;-3.55,-1.66,;-2.06,-2.04,;-1.64,-3.52,;-2.72,-4.63,;-4.21,-4.25,;-4.63,-2.76,;-2.3,-6.11,;-3.34,-7.24,;-2.87,-8.71,;-1.37,-9.04,;-.91,-10.51,;-.33,-7.91,;-.8,-6.44,;-5.49,.28,;-6.82,-.49,;-8.16,.28,;-8.16,1.82,;-6.82,2.59,;-6.82,4.13,;-5.49,1.82,)| | ||
| Structure | 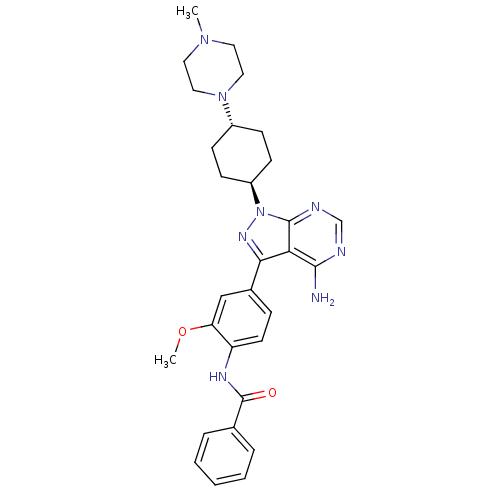
| ||