| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Genome polyprotein |
|---|
| Ligand | BDBM11243 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_158643 |
|---|
| IC50 | 23±n/a nM |
|---|
| Citation |  Jain, RP; Vederas, JC Structural variations in keto-glutamines for improved inhibition against hepatitis A virus 3C proteinase. Bioorg Med Chem Lett14:3655-8 (2004) [PubMed] Article Jain, RP; Vederas, JC Structural variations in keto-glutamines for improved inhibition against hepatitis A virus 3C proteinase. Bioorg Med Chem Lett14:3655-8 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Genome polyprotein |
|---|
| Name: | Genome polyprotein |
|---|
| Synonyms: | Human rhinovirus A protease | Human rhinovirus B 3A protease |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 44361.04 |
|---|
| Organism: | Human rhinovirus B |
|---|
| Description: | ChEMBL_158953 |
|---|
| Residue: | 401 |
|---|
| Sequence: | AFRPCNVNTKIGNAKCCPFVCGKAVTFKDRSTCSTYNLSSSLHHILEEDKRRRQVVDVMS
AIFQGPISLDAPPPPAIADLLQSVRTPRVIKYCQIIMGHPAECQVERDLNIANSIIAIIA
NIISIAGIIFVIYKLFCSLQGPYSGEPKPKTKVPERRVVAQGPEEEFGRSILKNNTCVIT
TGNGKFTGLGIHDRILIIPTHADPGREVQVNGVHTKVLDSYDLYNRDGVKLEITVIQLDR
NEKFRDIRKYIPETEDDYPECNLALSANQDEPTIIKVGDVVSYGNILLSGNQTARMLKYN
YPTKSGYCGGVLYKIGQILGIHVGGNGRDGFSAMLLRSYFTGQIKVNKHATECGLPDIQT
IHTPSKTKLQPSVFYDVFPGSKEPAVLTDNDPRLEVNFKEA
|
|
|
|---|
| BDBM11243 |
|---|
| n/a |
|---|
| Name | BDBM11243 |
|---|
| Synonyms: | AG7088 | CHEMBL20210 | US11859014, Compound rupintrivir | cmdc.202100576, 24f | ethyl (2E,4S)-4-[((2R,5S)-2-(4-fluorobenzyl)-6-methyl-5-{[(5-methylisoxazol-3-yl)carbonyl]amino}-4-oxoheptanoyl)amino]-5-[(3S)-2-oxopyrrolidin-3-yl]pent-2-enoate | ethyl (2E,4S)-4-[(2R,5S)-2-[(4-fluorophenyl)methyl]-6-methyl-5-[(5-methyl-1,2-oxazol-3-yl)formamido]-4-oxoheptanamido]-5-[(3S)-2-oxopyrrolidin-3-yl]pent-2-enoate | med.21724, Compound 29 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H39FN4O7 |
|---|
| Mol. Mass. | 598.6624 |
|---|
| SMILES | CCOC(=O)\C=C\[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@@H](CC(=O)[C@@H](NC(=O)c1cc(C)on1)C(C)C)Cc1ccc(F)cc1 |r| |
|---|
| Structure | 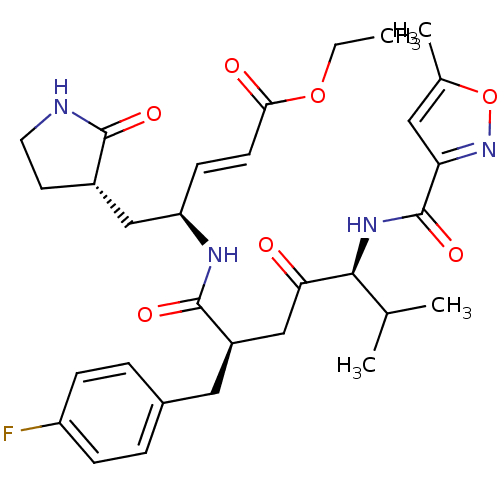
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jain, RP; Vederas, JC Structural variations in keto-glutamines for improved inhibition against hepatitis A virus 3C proteinase. Bioorg Med Chem Lett14:3655-8 (2004) [PubMed] Article
Jain, RP; Vederas, JC Structural variations in keto-glutamines for improved inhibition against hepatitis A virus 3C proteinase. Bioorg Med Chem Lett14:3655-8 (2004) [PubMed] Article