| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Telomerase reverse transcriptase |
|---|
| Ligand | BDBM50157551 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_430015 (CHEMBL917191) |
|---|
| IC50 | >40000±n/a nM |
|---|
| Citation |  Menichincheri, M; Ballinari, D; Bargiotti, A; Bonomini, L; Ceccarelli, W; D'Alessio, R; Fretta, A; Moll, J; Polucci, P; Soncini, C; Tibolla, M; Trosset, JY; Vanotti, E Catecholic flavonoids acting as telomerase inhibitors. J Med Chem47:6466-75 (2004) [PubMed] Article Menichincheri, M; Ballinari, D; Bargiotti, A; Bonomini, L; Ceccarelli, W; D'Alessio, R; Fretta, A; Moll, J; Polucci, P; Soncini, C; Tibolla, M; Trosset, JY; Vanotti, E Catecholic flavonoids acting as telomerase inhibitors. J Med Chem47:6466-75 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Telomerase reverse transcriptase |
|---|
| Name: | Telomerase reverse transcriptase |
|---|
| Synonyms: | EST2 | TCS1 | TERT | TERT_HUMAN | TRT |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 127099.03 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1447029 |
|---|
| Residue: | 1132 |
|---|
| Sequence: | MPRAPRCRAVRSLLRSHYREVLPLATFVRRLGPQGWRLVQRGDPAAFRALVAQCLVCVPW
DARPPPAAPSFRQVSCLKELVARVLQRLCERGAKNVLAFGFALLDGARGGPPEAFTTSVR
SYLPNTVTDALRGSGAWGLLLRRVGDDVLVHLLARCALFVLVAPSCAYQVCGPPLYQLGA
ATQARPPPHASGPRRRLGCERAWNHSVREAGVPLGLPAPGARRRGGSASRSLPLPKRPRR
GAAPEPERTPVGQGSWAHPGRTRGPSDRGFCVVSPARPAEEATSLEGALSGTRHSHPSVG
RQHHAGPPSTSRPPRPWDTPCPPVYAETKHFLYSSGDKEQLRPSFLLSSLRPSLTGARRL
VETIFLGSRPWMPGTPRRLPRLPQRYWQMRPLFLELLGNHAQCPYGVLLKTHCPLRAAVT
PAAGVCAREKPQGSVAAPEEEDTDPRRLVQLLRQHSSPWQVYGFVRACLRRLVPPGLWGS
RHNERRFLRNTKKFISLGKHAKLSLQELTWKMSVRDCAWLRRSPGVGCVPAAEHRLREEI
LAKFLHWLMSVYVVELLRSFFYVTETTFQKNRLFFYRKSVWSKLQSIGIRQHLKRVQLRE
LSEAEVRQHREARPALLTSRLRFIPKPDGLRPIVNMDYVVGARTFRREKRAERLTSRVKA
LFSVLNYERARRPGLLGASVLGLDDIHRAWRTFVLRVRAQDPPPELYFVKVDVTGAYDTI
PQDRLTEVIASIIKPQNTYCVRRYAVVQKAAHGHVRKAFKSHVSTLTDLQPYMRQFVAHL
QETSPLRDAVVIEQSSSLNEASSGLFDVFLRFMCHHAVRIRGKSYVQCQGIPQGSILSTL
LCSLCYGDMENKLFAGIRRDGLLLRLVDDFLLVTPHLTHAKTFLRTLVRGVPEYGCVVNL
RKTVVNFPVEDEALGGTAFVQMPAHGLFPWCGLLLDTRTLEVQSDYSSYARTSIRASLTF
NRGFKAGRNMRRKLFGVLRLKCHSLFLDLQVNSLQTVCTNIYKILLLQAYRFHACVLQLP
FHQQVWKNPTFFLRVISDTASLCYSILKAKNAGMSLGAKGAAGPLPSEAVQWLCHQAFLL
KLTRHRVTYVPLLGSLRTAQTQLSRKLPGTTLTALEAAANPALPSDFKTILD
|
|
|
|---|
| BDBM50157551 |
|---|
| n/a |
|---|
| Name | BDBM50157551 |
|---|
| Synonyms: | 6-(7,8-dimethoxy-4-oxo-4H-chromen-2-yl)-3-hydroxyquinoxalin-2(1H)-one | CHEMBL436311 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H14N2O6 |
|---|
| Mol. Mass. | 366.3243 |
|---|
| SMILES | COc1ccc2c(oc(cc2=O)-c2ccc3[nH]c(=O)c(=O)[nH]c3c2)c1OC |
|---|
| Structure | 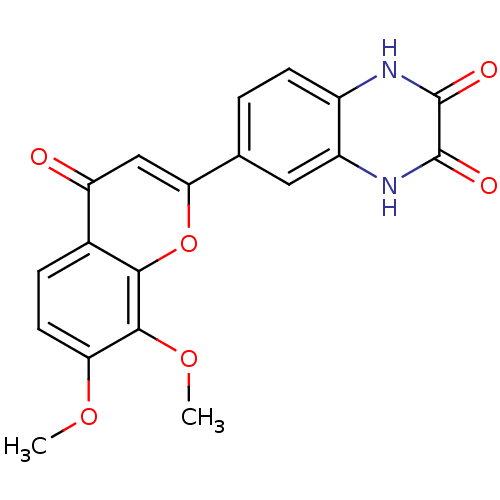
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Menichincheri, M; Ballinari, D; Bargiotti, A; Bonomini, L; Ceccarelli, W; D'Alessio, R; Fretta, A; Moll, J; Polucci, P; Soncini, C; Tibolla, M; Trosset, JY; Vanotti, E Catecholic flavonoids acting as telomerase inhibitors. J Med Chem47:6466-75 (2004) [PubMed] Article
Menichincheri, M; Ballinari, D; Bargiotti, A; Bonomini, L; Ceccarelli, W; D'Alessio, R; Fretta, A; Moll, J; Polucci, P; Soncini, C; Tibolla, M; Trosset, JY; Vanotti, E Catecholic flavonoids acting as telomerase inhibitors. J Med Chem47:6466-75 (2004) [PubMed] Article