| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2A |
|---|
| Ligand | BDBM50160806 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_303232 (CHEMBL827197) |
|---|
| Ki | 18.5±n/a nM |
|---|
| Citation |  Ochi, T; Sakamoto, M; Minamida, A; Suzuki, K; Ueda, T; Une, T; Toda, H; Matsumoto, K; Terauchi, Y Syntheses and properties of the major hydroxy metabolites in humans of blonanserin AD-5423, a novel antipsychotic agent. Bioorg Med Chem Lett15:1055-9 (2005) [PubMed] Article Ochi, T; Sakamoto, M; Minamida, A; Suzuki, K; Ueda, T; Une, T; Toda, H; Matsumoto, K; Terauchi, Y Syntheses and properties of the major hydroxy metabolites in humans of blonanserin AD-5423, a novel antipsychotic agent. Bioorg Med Chem Lett15:1055-9 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2A |
|---|
| Name: | 5-hydroxytryptamine receptor 2A |
|---|
| Synonyms: | 5-HT-2 | 5-HT-2A | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT-2A) | 5-hydroxytryptamine receptor 2A (5HT-2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_HUMAN | HTR2 | HTR2A | Serotonin receptor 2A |
|---|
| Type: | undefined |
|---|
| Mol. Mass.: | 52607.65 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P28223 |
|---|
| Residue: | 471 |
|---|
| Sequence: | MDILCEENTSLSSTTNSLMQLNDDTRLYSNDFNSGEANTSDAFNWTVDSENRTNLSCEGC
LSPSCLSLLHLQEKNWSALLTAVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIAD
MLLGFLVMPVSMLTILYGYRWPLPSKLCAVWIYLDVLFSTASIMHLCAISLDRYVAIQNP
IHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSF
VSFFIPLTIMVITYFLTIKSLQKEATLCVSDLGTRAKLASFSFLPQSSLSSEKLFQRSIH
REPGSYTGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNEDVIGA
LLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENKKPLQLILVNTIPALAYK
SSQLQMGQKKNSKQDAKTTDNDCSMVALGKQHSEEASKDNSDGVNEKVSCV
|
|
|
|---|
| BDBM50160806 |
|---|
| n/a |
|---|
| Name | BDBM50160806 |
|---|
| Synonyms: | 4-(4-Fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridin-7-ol | CHEMBL362285 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H30FN3O |
|---|
| Mol. Mass. | 383.5022 |
|---|
| SMILES | CCN1CCN(CC1)c1cc(-c2ccc(F)cc2)c2CC[C@@H](O)CCCc2n1 |
|---|
| Structure | 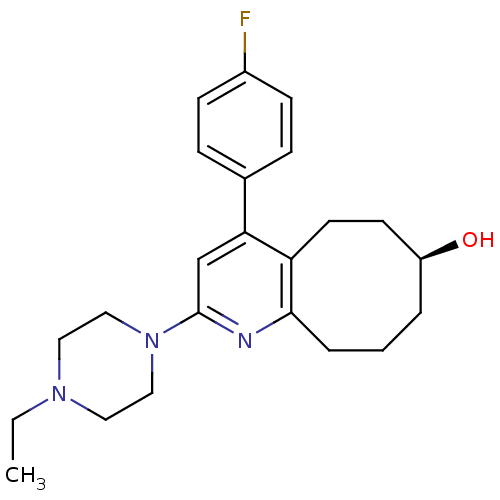
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ochi, T; Sakamoto, M; Minamida, A; Suzuki, K; Ueda, T; Une, T; Toda, H; Matsumoto, K; Terauchi, Y Syntheses and properties of the major hydroxy metabolites in humans of blonanserin AD-5423, a novel antipsychotic agent. Bioorg Med Chem Lett15:1055-9 (2005) [PubMed] Article
Ochi, T; Sakamoto, M; Minamida, A; Suzuki, K; Ueda, T; Une, T; Toda, H; Matsumoto, K; Terauchi, Y Syntheses and properties of the major hydroxy metabolites in humans of blonanserin AD-5423, a novel antipsychotic agent. Bioorg Med Chem Lett15:1055-9 (2005) [PubMed] Article