| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50168027 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_306024 (CHEMBL833539) |
|---|
| IC50 | 2200±n/a nM |
|---|
| Citation |  Migawa, MT; Drach, JC; Townsend, LB Design, synthesis and antiviral activity of novel 4,5-disubstituted 7-(beta-D-ribofuranosyl)pyrrolo[2,3-d][1,2,3]triazines and the novel 3-amino-5-methyl-1-(beta-D-ribofuranosyl)- and 3-amino-5-methyl-1-(2-deoxy-beta-D-ribofuranosyl)-1,5-dihydro-1,4,5,6,7,8-hexaazaacenaphthylene as analogues of tri J Med Chem48:3840-51 (2005) [PubMed] Article Migawa, MT; Drach, JC; Townsend, LB Design, synthesis and antiviral activity of novel 4,5-disubstituted 7-(beta-D-ribofuranosyl)pyrrolo[2,3-d][1,2,3]triazines and the novel 3-amino-5-methyl-1-(beta-D-ribofuranosyl)- and 3-amino-5-methyl-1-(2-deoxy-beta-D-ribofuranosyl)-1,5-dihydro-1,4,5,6,7,8-hexaazaacenaphthylene as analogues of tri J Med Chem48:3840-51 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50168027 |
|---|
| n/a |
|---|
| Name | BDBM50168027 |
|---|
| Synonyms: | (2R,3R,4S,5R)-2-(3-Amino-5-methyl-5H-1,4,5,6,7,8-hexaaza-acenaphthylen-1-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol | CHEMBL372715 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C12H15N7O4 |
|---|
| Mol. Mass. | 321.292 |
|---|
| SMILES | Cn1nc(N)c2cn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c3nnnc1c23 |
|---|
| Structure | 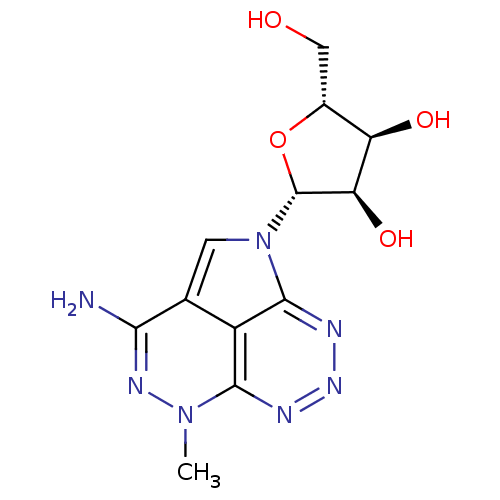
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Migawa, MT; Drach, JC; Townsend, LB Design, synthesis and antiviral activity of novel 4,5-disubstituted 7-(beta-D-ribofuranosyl)pyrrolo[2,3-d][1,2,3]triazines and the novel 3-amino-5-methyl-1-(beta-D-ribofuranosyl)- and 3-amino-5-methyl-1-(2-deoxy-beta-D-ribofuranosyl)-1,5-dihydro-1,4,5,6,7,8-hexaazaacenaphthylene as analogues of tri J Med Chem48:3840-51 (2005) [PubMed] Article
Migawa, MT; Drach, JC; Townsend, LB Design, synthesis and antiviral activity of novel 4,5-disubstituted 7-(beta-D-ribofuranosyl)pyrrolo[2,3-d][1,2,3]triazines and the novel 3-amino-5-methyl-1-(beta-D-ribofuranosyl)- and 3-amino-5-methyl-1-(2-deoxy-beta-D-ribofuranosyl)-1,5-dihydro-1,4,5,6,7,8-hexaazaacenaphthylene as analogues of tri J Med Chem48:3840-51 (2005) [PubMed] Article