| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prostacyclin receptor |
|---|
| Ligand | BDBM50594971 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2214835 (CHEMBL5127967) |
|---|
| EC50 | 1.9±n/a nM |
|---|
| Citation |  Chen, L; Yan, G; Ohwada, T Building on endogenous lipid mediators to design synthetic receptor ligands. Eur J Med Chem231:0 (2022) [PubMed] Article Chen, L; Yan, G; Ohwada, T Building on endogenous lipid mediators to design synthetic receptor ligands. Eur J Med Chem231:0 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prostacyclin receptor |
|---|
| Name: | Prostacyclin receptor |
|---|
| Synonyms: | PGI receptor | PI2R_HUMAN | PRIPR | PTGIR | Prostacyclin (IP) Receptor | Prostacyclin receptor | Prostaglandin I | Prostaglandin I2 | Prostaglandin I2 receptor | Prostanoid IP receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 40968.22 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | The membranes prepared from human platelet were used in binding assay. |
|---|
| Residue: | 386 |
|---|
| Sequence: | MADSCRNLTYVRGSVGPATSTLMFVAGVVGNGLALGILSARRPARPSAFAVLVTGLAATD
LLGTSFLSPAVFVAYARNSSLLGLARGGPALCDAFAFAMTFFGLASMLILFAMAVERCLA
LSHPYLYAQLDGPRCARLALPAIYAFCVLFCALPLLGLGQHQQYCPGSWCFLRMRWAQPG
GAAFSLAYAGLVALLVAAIFLCNGSVTLSLCRMYRQQKRHQGSLGPRPRTGEDEVDHLIL
LALMTVVMAVCSLPLTIRCFTQAVAPDSSSEMGDLLAFRFYAFNPILDPWVFILFRKAVF
QRLKLWVCCLCLGPAHGDSQTPLSQLASGRRDPRAPSAPVGKEGSCVPLSAWGEGQVEPL
PPTQQSSGSAVGTSSKAEASVACSLC
|
|
|
|---|
| BDBM50594971 |
|---|
| n/a |
|---|
| Name | BDBM50594971 |
|---|
| Synonyms: | 15-AU-81 | 15AU81 | CHEBI:50861 | L-606 | LRX -15 | LRX-15 | Remodulin | Rumodolin | TREPROSTINIL | Treprostinil | Tresprostinil | Tyvaso | Tyvaso dpi | UT-15 | Uniprost |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H34O5 |
|---|
| Mol. Mass. | 390.5131 |
|---|
| SMILES | [H][C@]12C[C@@H](O)[C@H](CC[C@@H](O)CCCCC)[C@@]1([H])Cc1cccc(OCC(O)=O)c1C2 |
|---|
| Structure | 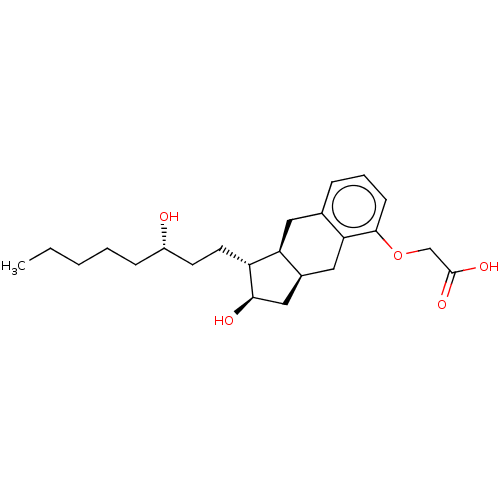
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chen, L; Yan, G; Ohwada, T Building on endogenous lipid mediators to design synthetic receptor ligands. Eur J Med Chem231:0 (2022) [PubMed] Article
Chen, L; Yan, G; Ohwada, T Building on endogenous lipid mediators to design synthetic receptor ligands. Eur J Med Chem231:0 (2022) [PubMed] Article