| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50170148 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_306441 (CHEMBL829085) |
|---|
| IC50 | >100000±n/a nM |
|---|
| Citation |  Normand-Bayle, M; Bénard, C; Zouhiri, F; Mouscadet, JF; Leh, H; Thomas, CM; Mbemba, G; Desmaële, D; d'Angelo, J New HIV-1 replication inhibitors of the styryquinoline class bearing aroyl/acyl groups at the C-7 position: synthesis and biological activity. Bioorg Med Chem Lett15:4019-22 (2005) [PubMed] Article Normand-Bayle, M; Bénard, C; Zouhiri, F; Mouscadet, JF; Leh, H; Thomas, CM; Mbemba, G; Desmaële, D; d'Angelo, J New HIV-1 replication inhibitors of the styryquinoline class bearing aroyl/acyl groups at the C-7 position: synthesis and biological activity. Bioorg Med Chem Lett15:4019-22 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50170148 |
|---|
| n/a |
|---|
| Name | BDBM50170148 |
|---|
| Synonyms: | CHEMBL188712 | {2-[(E)-2-(3,4-Dihydroxy-phenyl)-vinyl]-8-hydroxy-quinolin-7-yl}-(4-hydroxy-phenyl)-methanone |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H17NO5 |
|---|
| Mol. Mass. | 399.3955 |
|---|
| SMILES | Oc1ccc(cc1)C(=O)c1ccc2ccc(\C=C\c3ccc(O)c(O)c3)nc2c1O |
|---|
| Structure | 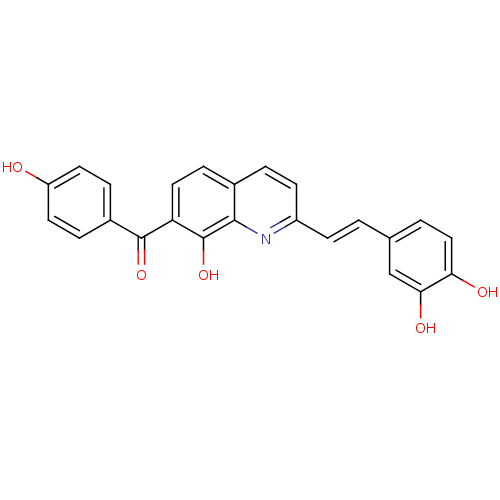
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Normand-Bayle, M; Bénard, C; Zouhiri, F; Mouscadet, JF; Leh, H; Thomas, CM; Mbemba, G; Desmaële, D; d'Angelo, J New HIV-1 replication inhibitors of the styryquinoline class bearing aroyl/acyl groups at the C-7 position: synthesis and biological activity. Bioorg Med Chem Lett15:4019-22 (2005) [PubMed] Article
Normand-Bayle, M; Bénard, C; Zouhiri, F; Mouscadet, JF; Leh, H; Thomas, CM; Mbemba, G; Desmaële, D; d'Angelo, J New HIV-1 replication inhibitors of the styryquinoline class bearing aroyl/acyl groups at the C-7 position: synthesis and biological activity. Bioorg Med Chem Lett15:4019-22 (2005) [PubMed] Article