| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholecystokinin receptor type A |
|---|
| Ligand | BDBM50202110 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_423414 (CHEMBL909382) |
|---|
| IC50 | 5.72±n/a nM |
|---|
| Citation |  Lee, YS; Agnes, RS; Davis, P; Ma, SW; Badghisi, H; Lai, J; Porreca, F; Hruby, VJ Partial retro-inverso, retro, and inverso modifications of hydrazide linked bifunctional peptides for opioid and cholecystokinin (CCK) receptors. J Med Chem50:165-8 (2007) [PubMed] Article Lee, YS; Agnes, RS; Davis, P; Ma, SW; Badghisi, H; Lai, J; Porreca, F; Hruby, VJ Partial retro-inverso, retro, and inverso modifications of hydrazide linked bifunctional peptides for opioid and cholecystokinin (CCK) receptors. J Med Chem50:165-8 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholecystokinin receptor type A |
|---|
| Name: | Cholecystokinin receptor type A |
|---|
| Synonyms: | CCK-A receptor | CCK-AR | CCK1-R | CCKAR | CCKAR_HUMAN | CCKRA | Cholecystokinin receptor | Cholecystokinin receptor type A | Cholecystokinin-1 Receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 47859.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Stable expression of human CCK-1 receptors in HEK 293 cells. |
|---|
| Residue: | 428 |
|---|
| Sequence: | MDVVDSLLVNGSNITPPCELGLENETLFCLDQPRPSKEWQPAVQILLYSLIFLLSVLGNT
LVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLKDFIFGSAVCKTTTYF
MGTSVSVSTFNLVAISLERYGAICKPLQSRVWQTKSHALKVIAATWCLSFTIMTPYPIYS
NLVPFTKNNNQTANMCRFLLPNDVMQQSWHTFLLLILFLIPGIVMMVAYGLISLELYQGI
KFEASQKKSAKERKPSTTSSGKYEDSDGCYLQKTRPPRKLELRQLSTGSSSRANRIRSNS
SAANLMAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTASAERRLSGTPISFILLLSY
TSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGARGEVGEEEEGGTTGASLSRFSYSH
MSASVPPQ
|
|
|
|---|
| BDBM50202110 |
|---|
| n/a |
|---|
| Name | BDBM50202110 |
|---|
| Synonyms: | CHEMBL218651 | H-Tyr-D-Ala-Gly-Phe-NH-NH-Trp-D-Nle-D-Asp-D-Phe-H |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C53H65N11O11 |
|---|
| Mol. Mass. | 1032.1503 |
|---|
| SMILES | CCCC[C@@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| |
|---|
| Structure | 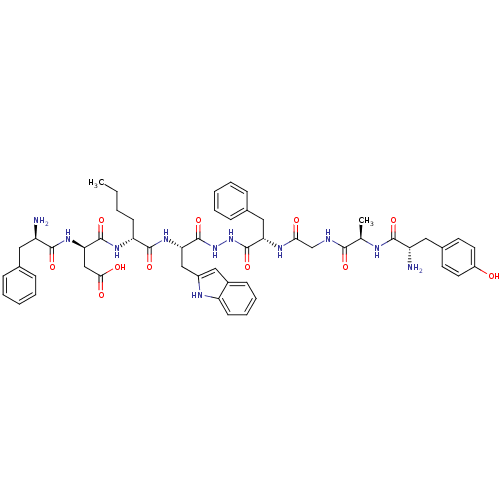
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Lee, YS; Agnes, RS; Davis, P; Ma, SW; Badghisi, H; Lai, J; Porreca, F; Hruby, VJ Partial retro-inverso, retro, and inverso modifications of hydrazide linked bifunctional peptides for opioid and cholecystokinin (CCK) receptors. J Med Chem50:165-8 (2007) [PubMed] Article
Lee, YS; Agnes, RS; Davis, P; Ma, SW; Badghisi, H; Lai, J; Porreca, F; Hruby, VJ Partial retro-inverso, retro, and inverso modifications of hydrazide linked bifunctional peptides for opioid and cholecystokinin (CCK) receptors. J Med Chem50:165-8 (2007) [PubMed] Article