| Reaction Details |
|---|
 | Report a problem with these data |
| Target | KiSS-1 receptor |
|---|
| Ligand | BDBM50203794 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_425686 (CHEMBL912002) |
|---|
| Ki | 1.8±n/a nM |
|---|
| Citation |  Orsini, MJ; Klein, MA; Beavers, MP; Connolly, PJ; Middleton, SA; Mayo, KH Metastin (KiSS-1) mimetics identified from peptide structure-activity relationship-derived pharmacophores and directed small molecule database screening. J Med Chem50:462-71 (2007) [PubMed] Article Orsini, MJ; Klein, MA; Beavers, MP; Connolly, PJ; Middleton, SA; Mayo, KH Metastin (KiSS-1) mimetics identified from peptide structure-activity relationship-derived pharmacophores and directed small molecule database screening. J Med Chem50:462-71 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| KiSS-1 receptor |
|---|
| Name: | KiSS-1 receptor |
|---|
| Synonyms: | AXOR12 | G-protein Coupled Receptor 54 | G-protein coupled receptor 54 (GPR54) | GPR54 | Hypogonadotropin-1 | KISS1R | KISSR_HUMAN | KiSS-1R | Kisspeptins receptor | Metastin receptor | hOT7T175 |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 42613.79 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Binding assay was performed using membranes from the CHO cell transfectants. |
|---|
| Residue: | 398 |
|---|
| Sequence: | MHTVATSGPNASWGAPANASGCPGCGANASDGPVPSPRAVDAWLVPLFFAALMLLGLVGN
SLVIYVICRHKPMRTVTNFYIANLAATDVTFLLCCVPFTALLYPLPGWVLGDFMCKFVNY
IQQVSVQATCATLTAMSVDRWYVTVFPLRALHRRTPRLALAVSLSIWVGSAAVSAPVLAL
HRLSPGPRAYCSEAFPSRALERAFALYNLLALYLLPLLATCACYAAMLRHLGRVAVRPAP
ADSALQGQVLAERAGAVRAKVSRLVAAVVLLFAACWGPIQLFLVLQALGPAGSWHPRSYA
AYALKTWAHCMSYSNSALNPLLYAFLGSHFRQAFRRVCPCAPRRPRRPRRPGPSDPAAPH
AELLRLGSHPAPARAQKPGSSGLAARGLCVLGEDNAPL
|
|
|
|---|
| BDBM50203794 |
|---|
| n/a |
|---|
| Name | BDBM50203794 |
|---|
| Synonyms: | (2S)-2-{[(2S)-1-[(2S)-2-amino-4-methylpentanoyl]pyrrolidin-2-yl]formamido}-N-[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(1S)-1-{[(1S)-4-carbamimidamido-1-{[(1S)-1-carbamoyl-2-phenylethyl]carbamoyl}butyl]carbamoyl}-3-methylbutyl]carbamoyl}methyl)carbamoyl]-2-phenylethyl]carbamoyl}-2-hydroxyethyl]carbamoyl}-2-carbamoylethyl]carbamoyl}ethyl]carbamoyl}-2-carbamoylethyl]carbamoyl}-2-(4-hydroxyphenyl)ethyl]butanediamide | CHEMBL221602 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C70H102N20O18 |
|---|
| Mol. Mass. | 1511.6821 |
|---|
| SMILES | [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#7])=O |
|---|
| Structure | 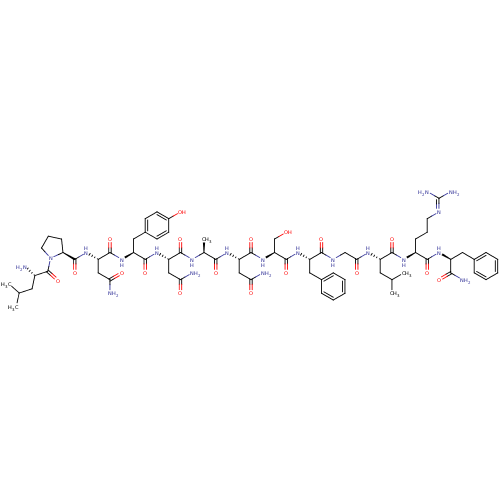
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Orsini, MJ; Klein, MA; Beavers, MP; Connolly, PJ; Middleton, SA; Mayo, KH Metastin (KiSS-1) mimetics identified from peptide structure-activity relationship-derived pharmacophores and directed small molecule database screening. J Med Chem50:462-71 (2007) [PubMed] Article
Orsini, MJ; Klein, MA; Beavers, MP; Connolly, PJ; Middleton, SA; Mayo, KH Metastin (KiSS-1) mimetics identified from peptide structure-activity relationship-derived pharmacophores and directed small molecule database screening. J Med Chem50:462-71 (2007) [PubMed] Article