| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Dual specificity protein phosphatase 22 |
|---|
| Ligand | BDBM50205436 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_438363 (CHEMBL887465) |
|---|
| IC50 | >40000±n/a nM |
|---|
| Citation |  Zhang, L; Qiu, B; Xiong, B; Li, X; Li, J; Wang, X; Li, J; Shen, J Quinoxalinylurea derivatives as a novel class of JSP-1 inhibitors. Bioorg Med Chem Lett17:2118-22 (2007) [PubMed] Article Zhang, L; Qiu, B; Xiong, B; Li, X; Li, J; Wang, X; Li, J; Shen, J Quinoxalinylurea derivatives as a novel class of JSP-1 inhibitors. Bioorg Med Chem Lett17:2118-22 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Dual specificity protein phosphatase 22 |
|---|
| Name: | Dual specificity protein phosphatase 22 |
|---|
| Synonyms: | DUS22_HUMAN | DUSP22 | Dual specificity phosphatase 22 | Dual specificity protein phosphatase (MKPX) | Dual specificity protein phosphatase 22 | Dual specificity protein phosphatase 22 (VHX) | Dual specificity protein phosphatase VHX (VHX) | JSP1 | LMWDSP2 | MKPX | Mitogen-activated protein kinase phosphatase x | VHX |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 20915.83 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q9NRW4 |
|---|
| Residue: | 184 |
|---|
| Sequence: | MGNGMNKILPGLYIGNFKDARDAEQLSKNKVTHILSVHDSARPMLEGVKYLCIPAADSPS
QNLTRHFKESIKFIHECRLRGESCLVHCLAGVSRSVTLVIAYIMTVTDFGWEDALHTVRA
GRSCANPNVGFQRQLQEFEKHEVHQYRQWLKEEYGESPLQDAEEAKNILAAPGILKFWAF
LRRL
|
|
|
|---|
| BDBM50205436 |
|---|
| n/a |
|---|
| Name | BDBM50205436 |
|---|
| Synonyms: | CHEMBL230362 | N-(2,3-bis(4-bromophenyl)quinoxalin-6-yl)-3-(pyrrolidine-1-carbonyl)piperidine-1-carboxamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H29Br2N5O2 |
|---|
| Mol. Mass. | 663.402 |
|---|
| SMILES | Brc1ccc(cc1)-c1nc2ccc(NC(=O)N3CCCC(C3)C(=O)N3CCCC3)cc2nc1-c1ccc(Br)cc1 |w:20.23| |
|---|
| Structure | 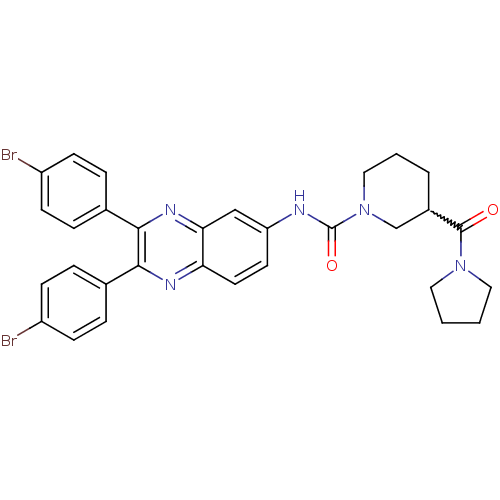
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhang, L; Qiu, B; Xiong, B; Li, X; Li, J; Wang, X; Li, J; Shen, J Quinoxalinylurea derivatives as a novel class of JSP-1 inhibitors. Bioorg Med Chem Lett17:2118-22 (2007) [PubMed] Article
Zhang, L; Qiu, B; Xiong, B; Li, X; Li, J; Wang, X; Li, J; Shen, J Quinoxalinylurea derivatives as a novel class of JSP-1 inhibitors. Bioorg Med Chem Lett17:2118-22 (2007) [PubMed] Article