| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2C9 |
|---|
| Ligand | BDBM50211853 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_436453 (CHEMBL904759) |
|---|
| IC50 | 37000±n/a nM |
|---|
| Citation |  Giblin, GM; O'Shaughnessy, CT; Naylor, A; Mitchell, WL; Eatherton, AJ; Slingsby, BP; Rawlings, DA; Goldsmith, P; Brown, AJ; Haslam, CP; Clayton, NM; Wilson, AW; Chessell, IP; Wittington, AR; Green, R Discovery of 2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro- 2H-pyran-4-yl)methyl]-4-(trifluoromethyl)- 5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem50:2597-600 (2007) [PubMed] Article Giblin, GM; O'Shaughnessy, CT; Naylor, A; Mitchell, WL; Eatherton, AJ; Slingsby, BP; Rawlings, DA; Goldsmith, P; Brown, AJ; Haslam, CP; Clayton, NM; Wilson, AW; Chessell, IP; Wittington, AR; Green, R Discovery of 2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro- 2H-pyran-4-yl)methyl]-4-(trifluoromethyl)- 5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem50:2597-600 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2C9 |
|---|
| Name: | Cytochrome P450 2C9 |
|---|
| Synonyms: | (R)-limonene 6-monooxygenase | (S)-limonene 6-monooxygenase | CP2C9_HUMAN | CYP2C10 | CYP2C9 | CYPIIC9 | Cytochrome P450 2C9 (CYP2C9 ) | Cytochrome P450 2C9 (CYP2C9) | P-450MP | P450 MP-4/MP-8 | P450 PB-1 | S-mephenytoin 4-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 55636.33 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11712 |
|---|
| Residue: | 490 |
|---|
| Sequence: | MDSLVVLVLCLSCLLLLSLWRQSSGRGKLPPGPTPLPVIGNILQIGIKDISKSLTNLSKV
YGPVFTLYFGLKPIVVLHGYEAVKEALIDLGEEFSGRGIFPLAERANRGFGIVFSNGKKW
KEIRRFSLMTLRNFGMGKRSIEDRVQEEARCLVEELRKTKASPCDPTFILGCAPCNVICS
IIFHKRFDYKDQQFLNLMEKLNENIKILSSPWIQICNNFSPIIDYFPGTHNKLLKNVAFM
KSYILEKVKEHQESMDMNNPQDFIDCFLMKMEKEKHNQPSEFTIESLENTAVDLFGAGTE
TTSTTLRYALLLLLKHPEVTAKVQEEIERVIGRNRSPCMQDRSHMPYTDAVVHEVQRYID
LLPTSLPHAVTCDIKFRNYLIPKGTTILISLTSVLHDNKEFPNPEMFDPHHFLDEGGNFK
KSKYFMPFSAGKRICVGEALAGMELFLFLTSILQNFNLKSLVDPKNLDTTPVVNGFASVP
PFYQLCFIPV
|
|
|
|---|
| BDBM50211853 |
|---|
| n/a |
|---|
| Name | BDBM50211853 |
|---|
| Synonyms: | 2-(2-fluoro-5-chlorophenylamino)-4-trifluoromethylpyrimidine-5-carboxylic acid(tetrahydro-pyran-4-ylmethyl)-amide | CHEMBL225464 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H17ClF4N4O2 |
|---|
| Mol. Mass. | 432.8 |
|---|
| SMILES | Fc1ccc(Cl)cc1Nc1ncc(C(=O)NCC2CCOCC2)c(n1)C(F)(F)F |
|---|
| Structure | 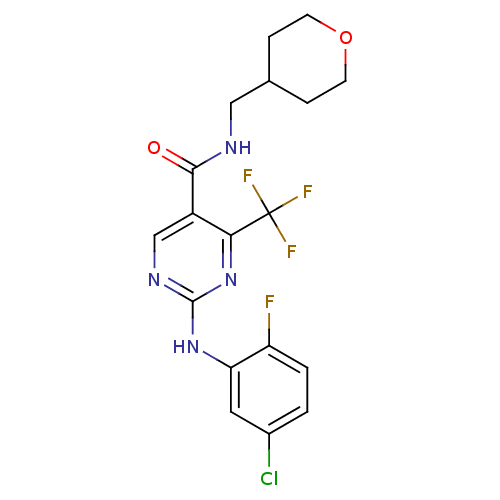
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Giblin, GM; O'Shaughnessy, CT; Naylor, A; Mitchell, WL; Eatherton, AJ; Slingsby, BP; Rawlings, DA; Goldsmith, P; Brown, AJ; Haslam, CP; Clayton, NM; Wilson, AW; Chessell, IP; Wittington, AR; Green, R Discovery of 2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro- 2H-pyran-4-yl)methyl]-4-(trifluoromethyl)- 5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem50:2597-600 (2007) [PubMed] Article
Giblin, GM; O'Shaughnessy, CT; Naylor, A; Mitchell, WL; Eatherton, AJ; Slingsby, BP; Rawlings, DA; Goldsmith, P; Brown, AJ; Haslam, CP; Clayton, NM; Wilson, AW; Chessell, IP; Wittington, AR; Green, R Discovery of 2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro- 2H-pyran-4-yl)methyl]-4-(trifluoromethyl)- 5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem50:2597-600 (2007) [PubMed] Article