| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prothrombin |
|---|
| Ligand | BDBM50215426 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_439444 (CHEMBL888564) |
|---|
| IC50 | >100000±n/a nM |
|---|
| Citation |  Trujillo, JI; Huang, HC; Neumann, WL; Mahoney, MW; Long, S; Huang, W; Garland, DJ; Kusturin, C; Abbas, Z; South, MS; Reitz, DB Design, synthesis, and biological evaluation of pyrazinones containing novel P1 needles as inhibitors of TF/VIIa. Bioorg Med Chem Lett17:4568-74 (2007) [PubMed] Article Trujillo, JI; Huang, HC; Neumann, WL; Mahoney, MW; Long, S; Huang, W; Garland, DJ; Kusturin, C; Abbas, Z; South, MS; Reitz, DB Design, synthesis, and biological evaluation of pyrazinones containing novel P1 needles as inhibitors of TF/VIIa. Bioorg Med Chem Lett17:4568-74 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prothrombin |
|---|
| Name: | Prothrombin |
|---|
| Synonyms: | Activation peptide fragment 1 | Activation peptide fragment 2 | Coagulation factor II | F2 | Prothrombin precursor | THRB_HUMAN | Thrombin heavy chain | Thrombin light chain |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 70029.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00734 |
|---|
| Residue: | 622 |
|---|
| Sequence: | MAHVRGLQLPGCLALAALCSLVHSQHVFLAPQQARSLLQRVRRANTFLEEVRKGNLEREC
VEETCSYEEAFEALESSTATDVFWAKYTACETARTPRDKLAACLEGNCAEGLGTNYRGHV
NITRSGIECQLWRSRYPHKPEINSTTHPGADLQENFCRNPDSSTTGPWCYTTDPTVRRQE
CSIPVCGQDQVTVAMTPRSEGSSVNLSPPLEQCVPDRGQQYQGRLAVTTHGLPCLAWASA
QAKALSKHQDFNSAVQLVENFCRNPDGDEEGVWCYVAGKPGDFGYCDLNYCEEAVEEETG
DGLDEDSDRAIEGRTATSEYQTFFNPRTFGSGEADCGLRPLFEKKSLEDKTERELLESYI
DGRIVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTEN
DLLVRIGKHSRTRYERNIEKISMLEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHP
VCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVLQVVNLPIVERPVCKDST
RIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKY
GFYTHVFRLKKWIQKVIDQFGE
|
|
|
|---|
| BDBM50215426 |
|---|
| n/a |
|---|
| Name | BDBM50215426 |
|---|
| Synonyms: | 2-[6-(3-aminophenyl)-5-chloro-3-(cyclobutylamino)-2-oxo-1,2-dihydropyrazin-1-yl]-N-[(4-carbamimidoyl-2,5,6-trifluoro-3-hydroxyphenyl)methyl]acetamide | CHEMBL230661 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H23ClF3N7O3 |
|---|
| Mol. Mass. | 549.933 |
|---|
| SMILES | NC(=N)c1c(O)c(F)c(CNC(=O)Cn2c(c(Cl)nc(NC3CCC3)c2=O)-c2cccc(N)c2)c(F)c1F |
|---|
| Structure | 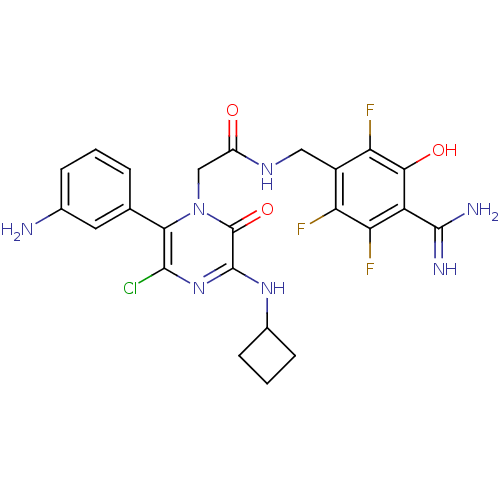
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Trujillo, JI; Huang, HC; Neumann, WL; Mahoney, MW; Long, S; Huang, W; Garland, DJ; Kusturin, C; Abbas, Z; South, MS; Reitz, DB Design, synthesis, and biological evaluation of pyrazinones containing novel P1 needles as inhibitors of TF/VIIa. Bioorg Med Chem Lett17:4568-74 (2007) [PubMed] Article
Trujillo, JI; Huang, HC; Neumann, WL; Mahoney, MW; Long, S; Huang, W; Garland, DJ; Kusturin, C; Abbas, Z; South, MS; Reitz, DB Design, synthesis, and biological evaluation of pyrazinones containing novel P1 needles as inhibitors of TF/VIIa. Bioorg Med Chem Lett17:4568-74 (2007) [PubMed] Article