| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50224181 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_450531 (CHEMBL900821) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Chen, C; Jiang, W; Tucci, F; Tran, JA; Fleck, BA; Hoare, SR; Joppa, M; Markison, S; Wen, J; Sai, Y; Johns, M; Madan, A; Chen, T; Chen, CW; Marinkovic, D; Arellano, M; Saunders, J; Foster, AC Discovery of 1-[2-[(1S)-(3-dimethylaminopropionyl)amino-2-methylpropyl]-4-methylphenyl]-4-[(2R)-methyl-3-(4-chlorophenyl)-propionyl]piperazine as an orally active antagonist of the melanocortin-4 receptor for the potential treatment of cachexia. J Med Chem50:5249-52 (2007) [PubMed] Article Chen, C; Jiang, W; Tucci, F; Tran, JA; Fleck, BA; Hoare, SR; Joppa, M; Markison, S; Wen, J; Sai, Y; Johns, M; Madan, A; Chen, T; Chen, CW; Marinkovic, D; Arellano, M; Saunders, J; Foster, AC Discovery of 1-[2-[(1S)-(3-dimethylaminopropionyl)amino-2-methylpropyl]-4-methylphenyl]-4-[(2R)-methyl-3-(4-chlorophenyl)-propionyl]piperazine as an orally active antagonist of the melanocortin-4 receptor for the potential treatment of cachexia. J Med Chem50:5249-52 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50224181 |
|---|
| n/a |
|---|
| Name | BDBM50224181 |
|---|
| Synonyms: | 1-{2-[(1S)-(3-dimethylaminopropionyl)amino-2-methylpropyl]-4-methylphenyl}-4-[(2R)-methyl-3-(4-chlorophenyl)propionyl]piperazine | CHEMBL427860 | N-((S)-1-(2-(4-((R)-3-(4-chlorophenyl)-2-methylpropanoyl)piperazin-1-yl)-5-methylphenyl)-2-methylpropyl)-3-(dimethylamino)propanamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H43ClN4O2 |
|---|
| Mol. Mass. | 527.141 |
|---|
| SMILES | CC(C)[C@H](NC(=O)CCN(C)C)c1cc(C)ccc1N1CCN(CC1)C(=O)[C@H](C)Cc1ccc(Cl)cc1 |r| |
|---|
| Structure | 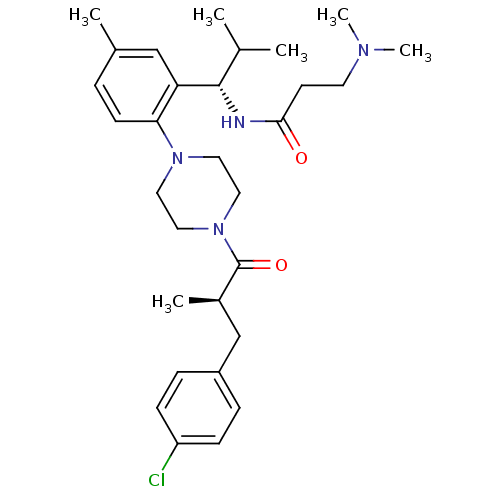
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chen, C; Jiang, W; Tucci, F; Tran, JA; Fleck, BA; Hoare, SR; Joppa, M; Markison, S; Wen, J; Sai, Y; Johns, M; Madan, A; Chen, T; Chen, CW; Marinkovic, D; Arellano, M; Saunders, J; Foster, AC Discovery of 1-[2-[(1S)-(3-dimethylaminopropionyl)amino-2-methylpropyl]-4-methylphenyl]-4-[(2R)-methyl-3-(4-chlorophenyl)-propionyl]piperazine as an orally active antagonist of the melanocortin-4 receptor for the potential treatment of cachexia. J Med Chem50:5249-52 (2007) [PubMed] Article
Chen, C; Jiang, W; Tucci, F; Tran, JA; Fleck, BA; Hoare, SR; Joppa, M; Markison, S; Wen, J; Sai, Y; Johns, M; Madan, A; Chen, T; Chen, CW; Marinkovic, D; Arellano, M; Saunders, J; Foster, AC Discovery of 1-[2-[(1S)-(3-dimethylaminopropionyl)amino-2-methylpropyl]-4-methylphenyl]-4-[(2R)-methyl-3-(4-chlorophenyl)-propionyl]piperazine as an orally active antagonist of the melanocortin-4 receptor for the potential treatment of cachexia. J Med Chem50:5249-52 (2007) [PubMed] Article