| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2D6 |
|---|
| Ligand | BDBM50198394 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_461650 (CHEMBL928768) |
|---|
| Ki | 600±n/a nM |
|---|
| Citation |  Watson, RJ; Allen, DR; Birch, HL; Chapman, GA; Galvin, FC; Jopling, LA; Knight, RL; Meier, D; Oliver, K; Meissner, JW; Owen, DA; Thomas, EJ; Tremayne, N; Williams, SC Development of CXCR3 antagonists. Part 3: Tropenyl and homotropenyl-piperidine urea derivatives. Bioorg Med Chem Lett18:147-51 (2008) [PubMed] Article Watson, RJ; Allen, DR; Birch, HL; Chapman, GA; Galvin, FC; Jopling, LA; Knight, RL; Meier, D; Oliver, K; Meissner, JW; Owen, DA; Thomas, EJ; Tremayne, N; Williams, SC Development of CXCR3 antagonists. Part 3: Tropenyl and homotropenyl-piperidine urea derivatives. Bioorg Med Chem Lett18:147-51 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2D6 |
|---|
| Name: | Cytochrome P450 2D6 |
|---|
| Synonyms: | CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55774.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10635 |
|---|
| Residue: | 497 |
|---|
| Sequence: | MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQ
LRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVF
LARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDK
AVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKV
LRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVA
DLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVI
HEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHF
LDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGV
FAFLVSPSPYELCAVPR
|
|
|
|---|
| BDBM50198394 |
|---|
| n/a |
|---|
| Name | BDBM50198394 |
|---|
| Synonyms: | (E)-1-(1-(cyclooctenylmethyl)piperidin-4-yl)-3-(naphthalen-2-yl)urea | 1-(1-(cyclooctenylmethyl)piperidin-4-yl)-3-(naphthalen-2-yl)urea | CHEMBL244610 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H33N3O |
|---|
| Mol. Mass. | 391.549 |
|---|
| SMILES | O=C(NC1CCN(C\C2=C\CCCCCC2)CC1)Nc1ccc2ccccc2c1 |t:8| |
|---|
| Structure | 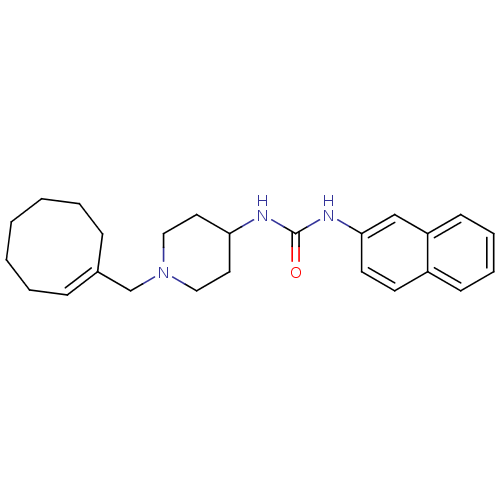
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Watson, RJ; Allen, DR; Birch, HL; Chapman, GA; Galvin, FC; Jopling, LA; Knight, RL; Meier, D; Oliver, K; Meissner, JW; Owen, DA; Thomas, EJ; Tremayne, N; Williams, SC Development of CXCR3 antagonists. Part 3: Tropenyl and homotropenyl-piperidine urea derivatives. Bioorg Med Chem Lett18:147-51 (2008) [PubMed] Article
Watson, RJ; Allen, DR; Birch, HL; Chapman, GA; Galvin, FC; Jopling, LA; Knight, RL; Meier, D; Oliver, K; Meissner, JW; Owen, DA; Thomas, EJ; Tremayne, N; Williams, SC Development of CXCR3 antagonists. Part 3: Tropenyl and homotropenyl-piperidine urea derivatives. Bioorg Med Chem Lett18:147-51 (2008) [PubMed] Article