

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227864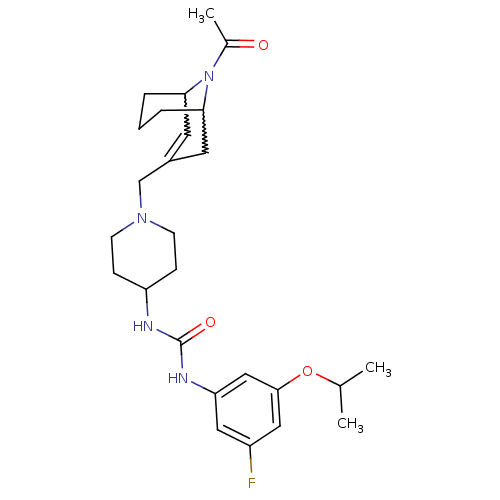 (1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227866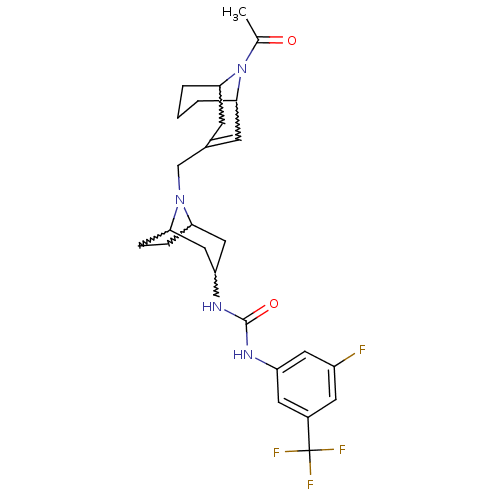 (1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227864 (1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227866 (1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227863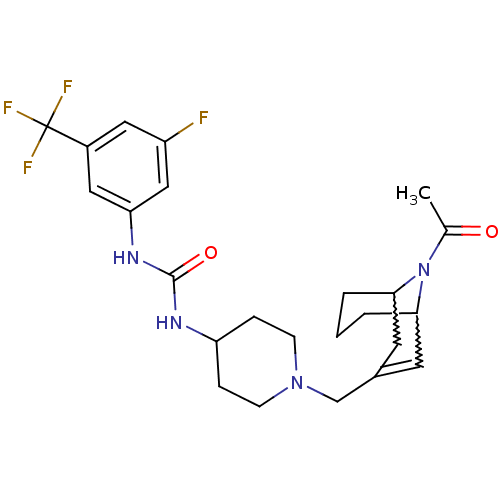 (1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227861 (1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((9-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227867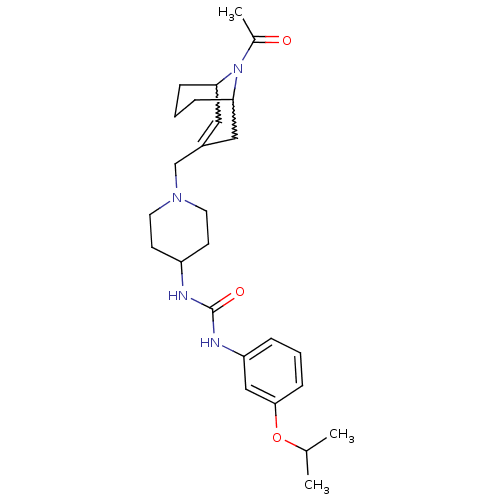 (1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227865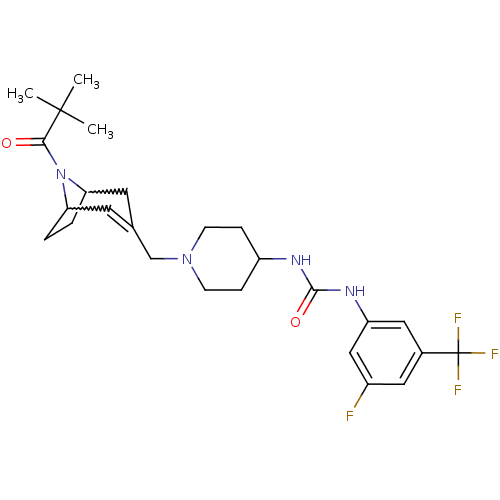 (1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((8-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227862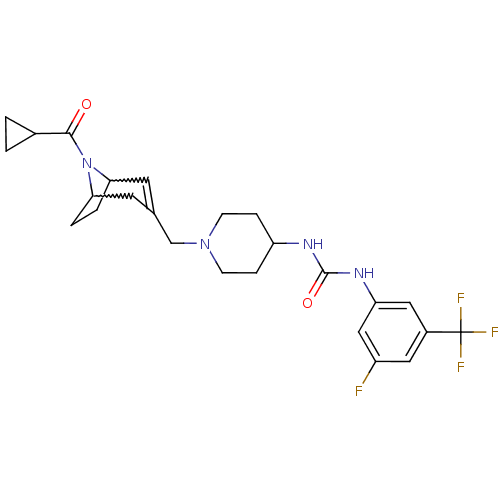 (1-(1-((8-(cyclopropanecarbonyl)-8-aza-bicyclo[3.2....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50198392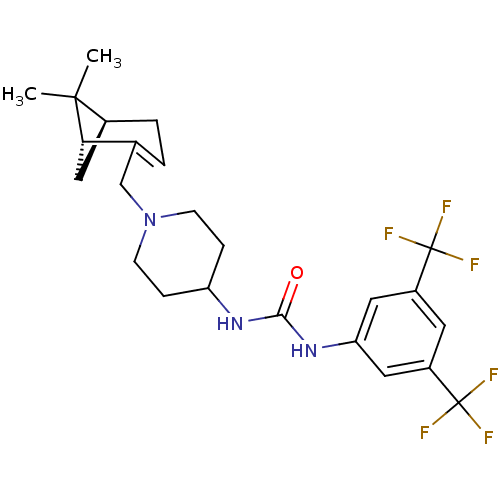 (1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-(((1R,5S)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227869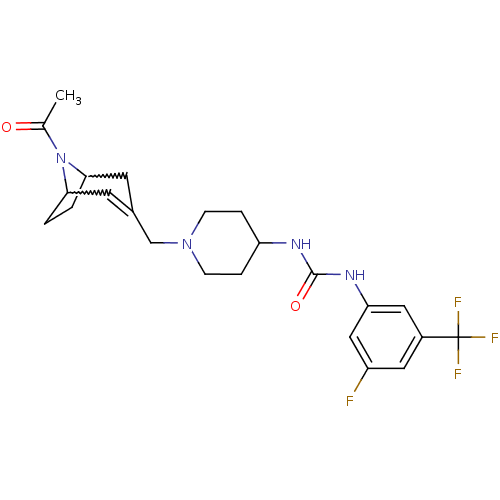 (1-[1-(8-acetyl-8-aza-bicyclo[3.2.1]oct-2-en-3-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227873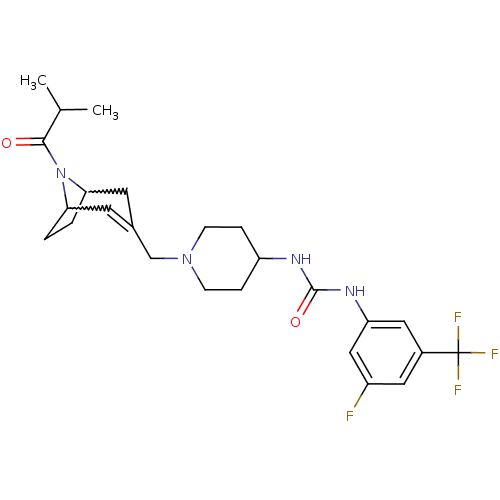 (1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((8-is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227867 (1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227863 (1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227861 (1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((9-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227868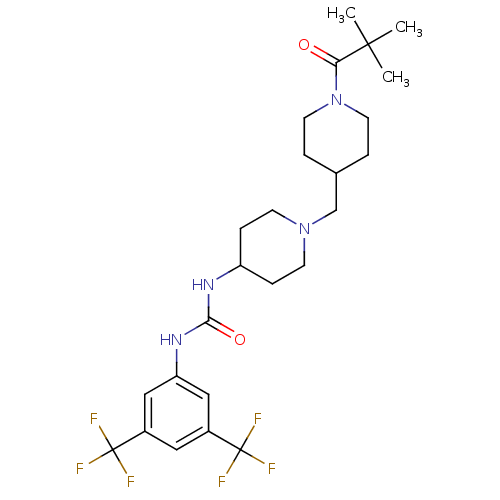 (1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-((1-pivalo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227862 (1-(1-((8-(cyclopropanecarbonyl)-8-aza-bicyclo[3.2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227865 (1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((8-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50198394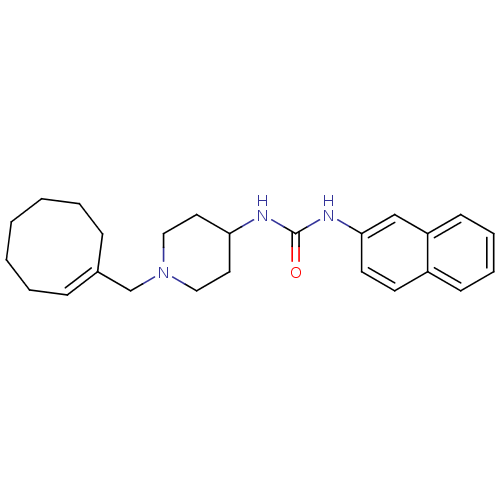 ((E)-1-(1-(cyclooctenylmethyl)piperidin-4-yl)-3-(na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227871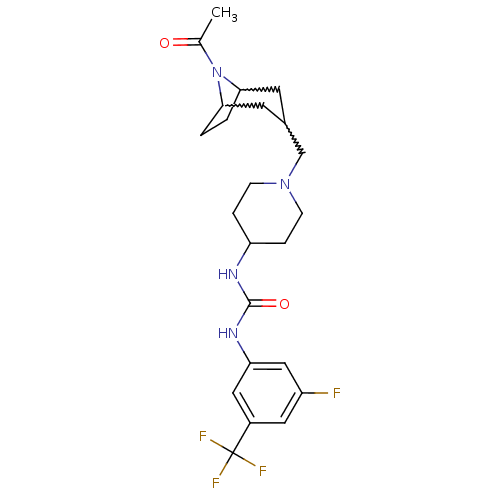 (1-(1-((8-acetyl-8-aza-bicyclo[3.2.1]octan-3-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227869 (1-[1-(8-acetyl-8-aza-bicyclo[3.2.1]oct-2-en-3-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227875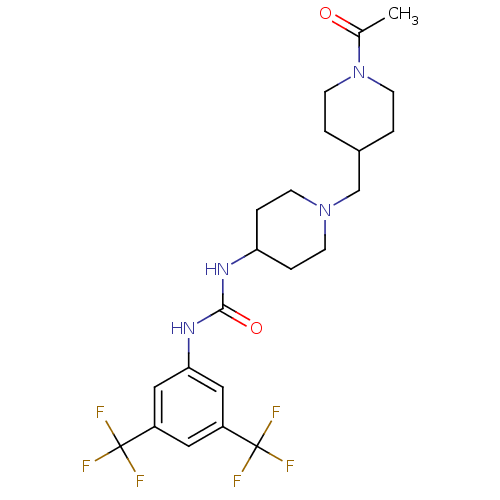 (1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-((1-acetyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227873 (1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((8-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227874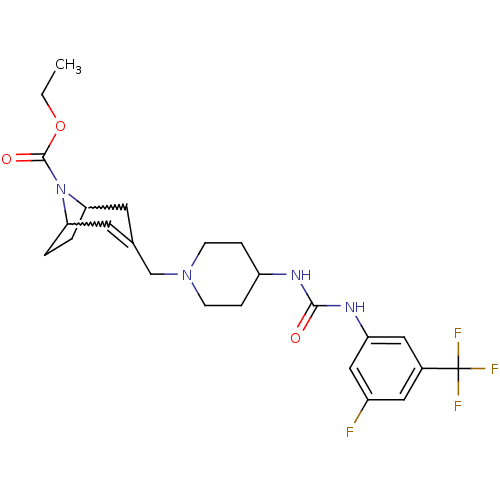 (3-{4-[3-(3-fluoro-5-trifluoromethyl-phenyl)-ureido...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50227866 (1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Inhibition of muscarinic M1 receptor | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227870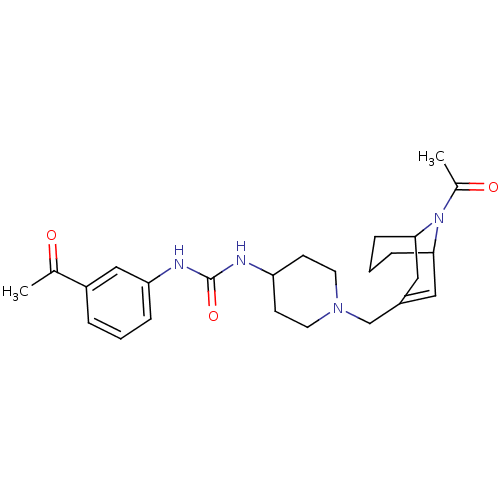 (1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50227866 (1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Inhibition of 5HT1A receptor | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50227866 (1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Inhibition of muscarinic M2 receptor | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50198392 (1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-(((1R,5S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50198394 ((E)-1-(1-(cyclooctenylmethyl)piperidin-4-yl)-3-(na...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50198394 ((E)-1-(1-(cyclooctenylmethyl)piperidin-4-yl)-3-(na...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227871 (1-(1-((8-acetyl-8-aza-bicyclo[3.2.1]octan-3-yl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50227874 (3-{4-[3-(3-fluoro-5-trifluoromethyl-phenyl)-ureido...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR3 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50227872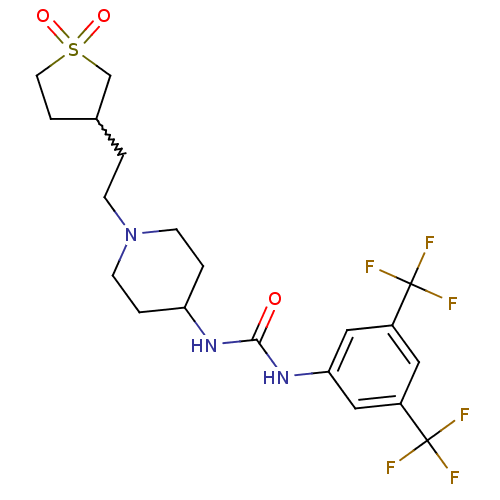 (1-(3,5-bis-trifluoromethyl-phenyl)-3-{1-[2-(1,1-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50227866 (1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Inhibition of muscarinic M3 receptor | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50227866 (1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Inhibition of 5HT5A receptor | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50198392 (1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-(((1R,5S)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50227866 (1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 18: 147-51 (2008) Article DOI: 10.1016/j.bmcl.2007.10.109 BindingDB Entry DOI: 10.7270/Q2MC8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103940 (US10399973, Example 7 | US8569338, 7 | US9035066, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | 25 |
Proximagen Ltd. US Patent | Assay Description All assays were performed in room temperature with purified recombinantly expressed human SSAO. The enzyme activity was measured with benzylamine as... | US Patent US8569338 (2013) BindingDB Entry DOI: 10.7270/Q2P849J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103940 (US10399973, Example 7 | US8569338, 7 | US9035066, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Proximagen Limited US Patent | Assay Description Briefly, test compounds were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 10 mM. Dose-response measurements were assayed by either cr... | US Patent US9035066 (2015) BindingDB Entry DOI: 10.7270/Q2G15ZKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103940 (US10399973, Example 7 | US8569338, 7 | US9035066, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Proximagen Limited US Patent | Assay Description Briefly, test compounds were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 10 mM. Dose-response measurements were assayed by either cr... | US Patent US9493457 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103940 (US10399973, Example 7 | US8569338, 7 | US9035066, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex | Assay Description Briefly, test compounds were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 10 mM. Dose-response measurements were assayed by either cr... | J Med Chem 51: 4986-99 (2008) BindingDB Entry DOI: 10.7270/Q2348NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103940 (US10399973, Example 7 | US8569338, 7 | US9035066, ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
BENEVOLENTAI CAMBRIDGE LIMITED US Patent | Assay Description All assays were performed in room temperature with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in Öhman... | US Patent US10399973 (2019) BindingDB Entry DOI: 10.7270/Q2PR7ZCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103941 (US10399973, Example 14 | US8569338, 14 | US9035066...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
BENEVOLENTAI CAMBRIDGE LIMITED US Patent | Assay Description All assays were performed in room temperature with purified recombinantly expressed human SSAO. Enzyme was prepared essentially as described in Öhman... | US Patent US10399973 (2019) BindingDB Entry DOI: 10.7270/Q2PR7ZCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103941 (US10399973, Example 14 | US8569338, 14 | US9035066...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | 25 |
Proximagen Ltd. US Patent | Assay Description All assays were performed in room temperature with purified recombinantly expressed human SSAO. The enzyme activity was measured with benzylamine as... | US Patent US8569338 (2013) BindingDB Entry DOI: 10.7270/Q2P849J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103941 (US10399973, Example 14 | US8569338, 14 | US9035066...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex | Assay Description Briefly, test compounds were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 10 mM. Dose-response measurements were assayed by either cr... | J Med Chem 51: 4986-99 (2008) BindingDB Entry DOI: 10.7270/Q2348NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103941 (US10399973, Example 14 | US8569338, 14 | US9035066...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Proximagen Limited US Patent | Assay Description Briefly, test compounds were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 10 mM. Dose-response measurements were assayed by either cr... | US Patent US9035066 (2015) BindingDB Entry DOI: 10.7270/Q2G15ZKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103941 (US10399973, Example 14 | US8569338, 14 | US9035066...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Proximagen Limited US Patent | Assay Description Briefly, test compounds were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 10 mM. Dose-response measurements were assayed by either cr... | US Patent US9493457 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103942 (US10399973, Example 17 | US8569338, 24 | US9035066...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex | Assay Description Briefly, test compounds were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 10 mM. Dose-response measurements were assayed by either cr... | J Med Chem 51: 4986-99 (2008) BindingDB Entry DOI: 10.7270/Q2348NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane primary amine oxidase (Homo sapiens (Human)) | BDBM103942 (US10399973, Example 17 | US8569338, 24 | US9035066...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Proximagen Limited US Patent | Assay Description Briefly, test compounds were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 10 mM. Dose-response measurements were assayed by either cr... | US Patent US9493457 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 77 total ) | Next | Last >> |