| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor gamma |
|---|
| Ligand | BDBM50229219 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_461781 (CHEMBL927787) |
|---|
| EC50 | 600±n/a nM |
|---|
| Citation |  Faucher, N; Martres, P; Laroze, A; Pineau, O; Potvain, F; Grillot, D Design, synthesis and evaluation of trifluoromethane sulfonamide derivatives as new potent and selective peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett18:710-5 (2008) [PubMed] Article Faucher, N; Martres, P; Laroze, A; Pineau, O; Potvain, F; Grillot, D Design, synthesis and evaluation of trifluoromethane sulfonamide derivatives as new potent and selective peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett18:710-5 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor gamma |
|---|
| Name: | Peroxisome proliferator-activated receptor gamma |
|---|
| Synonyms: | Nr1c3 | Nuclear receptor subfamily 1 group C member 3 | PPAR-gamma | PPARG_MOUSE | Pparg |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 57593.55 |
|---|
| Organism: | Mus musculus |
|---|
| Description: | ChEMBL_1453700 |
|---|
| Residue: | 505 |
|---|
| Sequence: | MGETLGDSPVDPEHGAFADALPMSTSQEITMVDTEMPFWPTNFGISSVDLSVMEDHSHSF
DIKPFTTVDFSSISAPHYEDIPFTRADPMVADYKYDLKLQEYQSAIKVEPASPPYYSEKT
QLYNRPHEEPSNSLMAIECRVCGDKASGFHYGVHACEGCKGFFRRTIRLKLIYDRCDLNC
RIHKKSRNKCQYCRFQKCLAVGMSHNAIRFGRMPQAEKEKLLAEISSDIDQLNPESADLR
ALAKHLYDSYIKSFPLTKAKARAILTGKTTDKSPFVIYDMNSLMMGEDKIKFKHITPLQE
QSKEVAIRIFQGCQFRSVEAVQEITEYAKNIPGFINLDLNDQVTLLKYGVHEIIYTMLAS
LMNKDGVLISEGQGFMTREFLKNLRKPFGDFMEPKFEFAVKFNALELDDSDLAIFIAVII
LSGDRPGLLNVKPIEDIQDNLLQALELQLKLNHPESSQLFAKVLQKMTDLRQIVTEHVQL
LHVIKKTETDMSLHPLLQEIYKDLY
|
|
|
|---|
| BDBM50229219 |
|---|
| n/a |
|---|
| Name | BDBM50229219 |
|---|
| Synonyms: | 4'-chloro-biphenyl-4-carboxylic acid 4-trifluoromethanesulfonylamino-benzylamide | CHEMBL252675 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H16ClF3N2O3S |
|---|
| Mol. Mass. | 468.877 |
|---|
| SMILES | FC(F)(F)S(=O)(=O)Nc1ccc(CNC(=O)c2ccc(cc2)-c2ccc(Cl)cc2)cc1 |
|---|
| Structure | 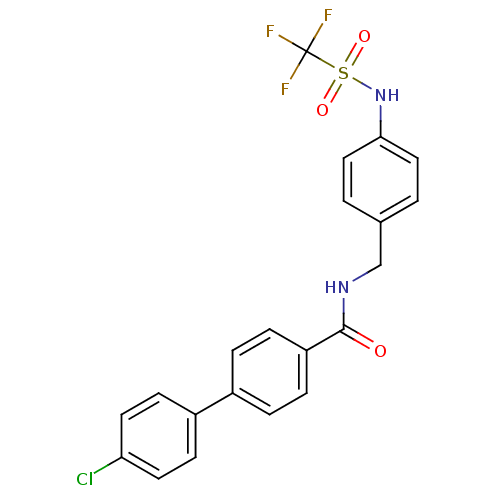
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Faucher, N; Martres, P; Laroze, A; Pineau, O; Potvain, F; Grillot, D Design, synthesis and evaluation of trifluoromethane sulfonamide derivatives as new potent and selective peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett18:710-5 (2008) [PubMed] Article
Faucher, N; Martres, P; Laroze, A; Pineau, O; Potvain, F; Grillot, D Design, synthesis and evaluation of trifluoromethane sulfonamide derivatives as new potent and selective peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett18:710-5 (2008) [PubMed] Article