| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor alpha |
|---|
| Ligand | BDBM50229223 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_461773 (CHEMBL927779) |
|---|
| EC50 | 20±n/a nM |
|---|
| Citation |  Faucher, N; Martres, P; Laroze, A; Pineau, O; Potvain, F; Grillot, D Design, synthesis and evaluation of trifluoromethane sulfonamide derivatives as new potent and selective peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett18:710-5 (2008) [PubMed] Article Faucher, N; Martres, P; Laroze, A; Pineau, O; Potvain, F; Grillot, D Design, synthesis and evaluation of trifluoromethane sulfonamide derivatives as new potent and selective peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett18:710-5 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor alpha |
|---|
| Name: | Peroxisome proliferator-activated receptor alpha |
|---|
| Synonyms: | NR1C1 | Nuclear receptor subfamily 1 group C member 1 | PPAR | PPAR alpha/gamma | PPAR-alpha | PPARA | PPARA_HUMAN | Peroxisome Proliferator-Activated Receptor alpha | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor alpha (PPAR alpha) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 52222.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q07869 |
|---|
| Residue: | 468 |
|---|
| Sequence: | MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSC
PGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACE
GCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSE
KAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFV
IHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANL
DLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFD
FAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDI
FLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY
|
|
|
|---|
| BDBM50229223 |
|---|
| n/a |
|---|
| Name | BDBM50229223 |
|---|
| Synonyms: | 4'-cyano-biphenyl-4-carboxylic acid 4-trifluoromethanesulfonylamino-bezylamide | CHEMBL253486 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H16F3N3O3S |
|---|
| Mol. Mass. | 459.441 |
|---|
| SMILES | FC(F)(F)S(=O)(=O)Nc1ccc(CNC(=O)c2ccc(cc2)-c2ccc(cc2)C#N)cc1 |
|---|
| Structure | 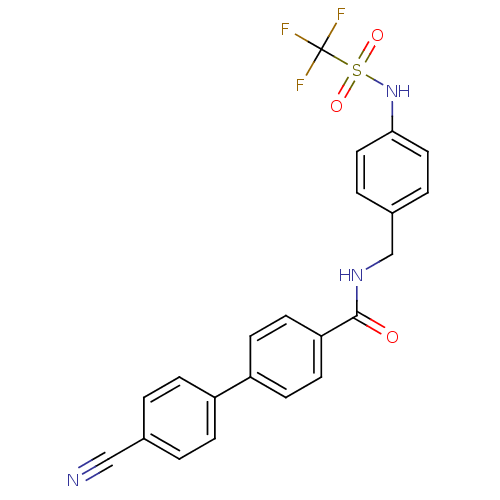
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Faucher, N; Martres, P; Laroze, A; Pineau, O; Potvain, F; Grillot, D Design, synthesis and evaluation of trifluoromethane sulfonamide derivatives as new potent and selective peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett18:710-5 (2008) [PubMed] Article
Faucher, N; Martres, P; Laroze, A; Pineau, O; Potvain, F; Grillot, D Design, synthesis and evaluation of trifluoromethane sulfonamide derivatives as new potent and selective peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett18:710-5 (2008) [PubMed] Article