| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-X-C chemokine receptor type 3 |
|---|
| Ligand | BDBM50229376 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_461808 (CHEMBL928937) |
|---|
| IC50 | 11±n/a nM |
|---|
| Citation |  Li, AR; Johnson, MG; Liu, J; Chen, X; Du, X; Mihalic, JT; Deignan, J; Gustin, DJ; Duquette, J; Fu, Z; Zhu, L; Marcus, AP; Bergeron, P; McGee, LR; Danao, J; Lemon, B; Carabeo, T; Sullivan, T; Ma, J; Tang, L; Tonn, G; Collins, TL; Medina, JC Optimization of the heterocyclic core of the quinazolinone-derived CXCR3 antagonists. Bioorg Med Chem Lett18:688-93 (2008) [PubMed] Article Li, AR; Johnson, MG; Liu, J; Chen, X; Du, X; Mihalic, JT; Deignan, J; Gustin, DJ; Duquette, J; Fu, Z; Zhu, L; Marcus, AP; Bergeron, P; McGee, LR; Danao, J; Lemon, B; Carabeo, T; Sullivan, T; Ma, J; Tang, L; Tonn, G; Collins, TL; Medina, JC Optimization of the heterocyclic core of the quinazolinone-derived CXCR3 antagonists. Bioorg Med Chem Lett18:688-93 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-X-C chemokine receptor type 3 |
|---|
| Name: | C-X-C chemokine receptor type 3 |
|---|
| Synonyms: | AAO92295.1 | C-X-C chemokine receptor type 3 | C-X-C chemokine receptor type 3 (CXCR3) | C-X-C chemokine receptor type 3 (CXCR3A) | CXCR3 | CXCR3A | CXCR3_HUMAN | GPR9 | chemokine (C-X-C motif) receptor 3 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 40665.65 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 368 |
|---|
| Sequence: | MVLEVSDHQVLNDAEVAALLENFSSSYDYGENESDSCCTSPPCPQDFSLNFDRAFLPALY
SLLFLLGLLGNGAVAAVLLSRRTALSSTDTFLLHLAVADTLLVLTLPLWAVDAAVQWVFG
SGLCKVAGALFNINFYAGALLLACISFDRYLNIVHATQLYRRGPPARVTLTCLAVWGLCL
LFALPDFIFLSAHHDERLNATHCQYNFPQVGRTALRVLQLVAGFLLPLLVMAYCYAHILA
VLLVSRGQRRLRAMRLVVVVVVAFALCWTPYHLVVLVDILMDLGALARNCGRESRVDVAK
SVTSGLGYMHCCLNPLLYAFVGVKFRERMWMLLLRLGCPNQRGLQRQPSSSRRDSSWSET
SEASYSGL
|
|
|
|---|
| BDBM50229376 |
|---|
| n/a |
|---|
| Name | BDBM50229376 |
|---|
| Synonyms: | (R)-N-(1-(3-(4-cyanophenyl)-4-oxo-3,4-dihydropyrido[2,3-d]pyrimidin-2-yl)ethyl)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trifluoromethyl)phenyl)acetamide | CHEMBL253431 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H25F4N5O4S |
|---|
| Mol. Mass. | 615.599 |
|---|
| SMILES | CCS(=O)(=O)CCN([C@H](C)c1nc2ncccc2c(=O)n1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F |
|---|
| Structure | 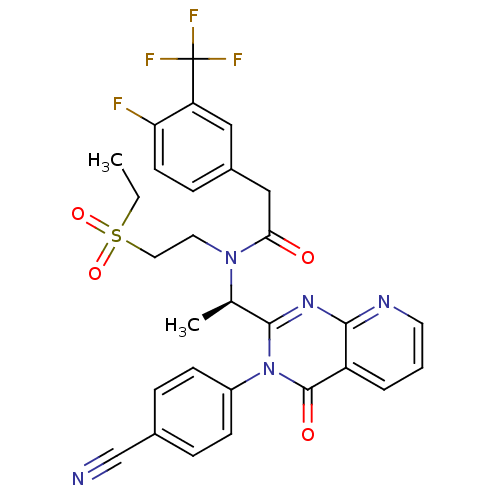
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Li, AR; Johnson, MG; Liu, J; Chen, X; Du, X; Mihalic, JT; Deignan, J; Gustin, DJ; Duquette, J; Fu, Z; Zhu, L; Marcus, AP; Bergeron, P; McGee, LR; Danao, J; Lemon, B; Carabeo, T; Sullivan, T; Ma, J; Tang, L; Tonn, G; Collins, TL; Medina, JC Optimization of the heterocyclic core of the quinazolinone-derived CXCR3 antagonists. Bioorg Med Chem Lett18:688-93 (2008) [PubMed] Article
Li, AR; Johnson, MG; Liu, J; Chen, X; Du, X; Mihalic, JT; Deignan, J; Gustin, DJ; Duquette, J; Fu, Z; Zhu, L; Marcus, AP; Bergeron, P; McGee, LR; Danao, J; Lemon, B; Carabeo, T; Sullivan, T; Ma, J; Tang, L; Tonn, G; Collins, TL; Medina, JC Optimization of the heterocyclic core of the quinazolinone-derived CXCR3 antagonists. Bioorg Med Chem Lett18:688-93 (2008) [PubMed] Article